Hypothalamic Neuropeptides in the Autonomic Innervation of Gingiva and Lip
Katalin Koves
Eniko Szabo1, Katalin Koves2*, Zsolt Boldogkoi3, Agnes Csaki2, Zsolt Lohinai1, Zsuzsanna Toth1 and Philippe Ciofi4
1Department of Conservative Dentistry, Faculty of Dentistry, Semmelweis University, Budapest, 1085-Hungary
2Department of Anatomy, Histology and Embryology, Faculty of Medicine, Semmelweis University, Budapest, 1094-Hungary
3Department of Medical Biology, Faculty of Medicine, University of Szeged, 6720-Hungary
4INSERM U862, Neurocentre Magendie, Bordeaux, France
- *Corresponding Author:
- Katalin Koves
Department of Anatomy, Histology and Embryology, Faculty of Medicine
Semmelweis niversity, Tuzolto u. 58, H-1094 Budapest, Hungary
Tel: 361215 6920
E-mail: koves.katalin@med
Received date: March 17, 2016; Accepted date: April 07, 2016; Published date: April 11, 2016
Citation: Szabo E, Koves K, Boldogkoi Z, et al. Hypothalamic Neuropeptides in the Autonomic Innervation of Gingiva and Lip. J Transl Neurosci. 2016, 1:1.
Copyright: © 2016 Szabo E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Ample evidence indicates that the descending pathways from the hypothalamus play a role in the autonomic regulation of the lower gingiva and lip. Our goal was to identify the neurochemical nature of the hypothalamic neurons giving origin of these multisynaptic descending pathways and additionally to characterize the lower members of these pathways. Methodology: Retrogradely spreading Green Fluorescence Protein (GFP) labeled virus was injected into the lower gingiva or lip of Wistar rats. Intact and sympathectomized rats were included in the experiment. Virus labeling was looked for in frozen sections of the hypothalamus, brainstem, upper thoracic spinal cord, superior cervical, otic and submandibular ganglia. Results: In intact and sympathectomized rats, labeled neurons of the hypothalamic paraventricular nucleus showed oxytocin, vasopressin, but not Cholecystokinin (CCK) and Corticotropic hormone Releasing Hormone (CRH) immunoreactivities. In the perifornical region the virus labeled neurons showed orexin immunoreactivitiy. In intact and sympathectomized rats the members of the descending pathway were further characterized. In sympathectomized rats the labeling was missing from the locus ceruleus, the ventrolateral medulla, the raphe nuclei, the spinal cord, but labeling further existed in the gigantocellular, the salivatory nuclei and the hypothalamus suggesting their parasympathetic nature. Conclusion: Our paper demonstrates for the first time that oxytocin, vasopressin and orexin, but not CCK and CRH, immunoreactive hypothalamic neurons may influence both sympathetic and parasympathetic responses of the lower gingiva and lip. These are common command neurons. We also summarized the data step-by-step on the chemical characteristics of the lower part of the descending pathway.
Keywords
Transneuronal virus; Tracing technique; Immunohistochemistry; Neurotransmitters; Neuropeptides; Double labeling; Confocal microscopy; Rats
Abbreviations
DBH: Dopamine β-Hydroxylase; CGRP: Calcitonin Gene-Related Peptide; CCK: Cholecystokinin; CRH: Corticotropic Hormone Releasing Hormone; G: Gingiva; Gi: Gigantocellular Reticular Nucleus; GFP: Green Fluorescence Protein; HRP/WGA: Horseradish Peroxidase/Wheat Germ Agglutinin; IML: Intermediolateral Cell Column; ISN: Inferior Salivatory Nucleus; KPB: Potassium Phosphate Buffer; L: Lip; LC: Locus Ceruleus; L-ENK: Leu-Enkephalin; NOS: Nitric Oxide Synthase; NPY: Neuropeptide-Y; OG: Otic Ganglion; ORX: Orexin; OT: Oxytocin; OT-N: Oxytocin- Neurophysin; Pf : Perifornical Region; Rmg: Raphe Magnus; Rpa: Raphe Pallidus; PRV: Pseudorabies Virus; PVN: Paraventricular Nucleus; SCG: Superior Cervical Ganglion; SER: Serotonin; SMG: Submandibular Ganglion; SP: Substance P; SS: Somatostatin; SSN: Superior Salivatiry Nucleus; TriG: Trigeminal Ganglion; VAChT: Vesicular Acetylcholine Transporter; VIP: Vasoactive Intestinal Polypeptide; VLM: Ventrolateral Medulla; rVLM: Rostral Ventrolateral Medulla; VP: Vasopressin; VPN: Vasopressin Neurophysin
Introduction
The transneuronal virus labeling is a suitable method to trace neural pathways which innervate peripheral target organs. It was found two decades ago that the pseudorabies virus could only be transported by neurons. After replication in a neuronal cell body, the virus can enter the next member of the neuronal chain and is able to demonstrate neuronal [1].
It is well known that the postganglionic parasympathetic fibers innervating the gingiva (G) or lip (L) tend to join the sensory or motor nerves [2]. In the human G the nerve fibers contain calcitonin Gene-Related Peptide (CGRP), Substance-P (SP), Vasoactive Intestinal Peptide (VIP) and Neuropeptide-Y (NPY). The presence of NPY and VIP fibers occasionally observed around blood vessels of the G suggests that they are autonomic in nature [3]. Another research group demonstrated noradrenergic fibers in the lamina propria and in the wall of the vessels [4]. Horseradish Peroxidase/Wheat Germ Agglutinin (HRP/WGA) administration into the molar gland and the lower lip-gingiva of cat showed retrogradely labeled neurons in the Otic Ganglion (OG), Superior Cervical Ganglion (SCG) and mandibular subdivision of the Trigeminal Ganglia (TriG) [5]. This observation suggests that the nerve fibers found in the gingiva and lip derive from these sources. It was also demonstrated that the mucous membrane of the human lip contains fibers immunoreactive for VIP, SP, NPY and Nitric Oxide Synthase (NOS) [6]. Lohinai et al. [7] described NOS immunoreactive fibers in cat and dog gingivae.
Numerous data accumulated in the literature on the location of neurons in the central nervous system which are involved in the autonomic innervation of many viscera including the suprarenal gland the ovary, the kidney, the colon, the penis, the prostate gland and the perineal muscles, the urinary bladder, the salivary and mammary glands [8-20]. As it was predicted by Strack et al. [21], who injected a retrograde pseudorabies virus into various sympathetic ganglia and the adrenal gland of rats, the premotor sympathetic neurons are generally found in several cell groups that regulate the entire sympathetic outflow: the hypothalamic Paraventricular Nucleus (PVN), perifornical region (Pf), A5 noradrenergic cell group, caudal raphe region, rostral Ventrolateral Medulla (VLM), the Locus Ceruleus (LC) and the Barrington's nucleus. Additionally, local interneurons in laminae VII and X of the spinal cord are also involved in the neuronal chain innervating the preganglionic neurons in the Intermediolateral cell group (IML). They also showed that the retrograde transneuronal viral labeling method could be used simultaneously with either neuropeptide or neurotransmitter synthetic enzyme immunohistochemistry.
Only a few data are available in the literature on the neuropeptides, located in the hypothalamic PVN and Pf, and on those involved in the regulation of the autonomic responses of peripheral organs. We observed that a retrograde spreading trans-synaptic virus injected in the mammary gland appeared in the PVN and the Pf. A subpopulation of the virus labeled cells in the PVN contained Oxytocin (OT) [20].
The major goal of this present study was to examine the chemical nature of the neurons, located in the PVN and Pf sending autonomic information through the brainstem and the spinal cord to the lower G and L. The lower members of the descending pathway were also characterized. We have used transneuronal retrograde tracing technique and neuropeptide immunohistochemistry. We carried out OT, Vasopressin (VP), Cholecystokinin (CCK) and Corticotropic hormone Releasing Hormone (CRH) immunostaining in the hypothalamic sections containing PVN. The presence of virus labeling in the Pf suggested that the virus labeling might also be present in Orexin (ORX) immunoreactive cells which were discovered about two decades ago [22-24].
In a part of the animals sympathectomy was carried out. This intervention gave us the possibility to investigate separately the retrograde projection of the parasympathetic innervation of the lower G and L. The reason why we examined these animal groups was because studies demonstrated neurons in PVN that are common command neurons of both sympathetic and parasympathetic regulation of the submandibular gland.
Materials and Method
Animals
Adult Wistar female rats (3-4 month old) were used for the experiments. The animals were kept in a light/dark cycle (lights on at 5:00 and lights off at 19:00) and temperature controlled vivarium (22 ± 2°C). The treatment of the animals was in accordance with the rules of the “European convention for the protection of vertebrate animals used for experimental and other scientific purposes”, Strasbourg, 1986 and The Hungarian Government Directive 243/98. Our protocol was approved by the Local Animal Care and Use Committee (Permission No: 22.1/1158/3/2010).
Preparing and injection of the virus
The same genetically modified Pseudorabies Virus (PRV) strain termed memGreen-PRV was used for the experiments as in our previous paper [25]. The construction of memGreen-PRV was previously described [25-27]. The virus expressed Green Gluorescence Protein (GFP) and the presence of virus was easily discovered using fluorescence microscope. The concentration of the virus was 8 × 108/mL plaque forming units. The spreading speed of the virus in a retrograde direction was 1,5 mm/h. Under general anesthesia (chloralhydrate 35 mg/100 gr bw), with the use of 10 μL Hamilton syringe, 4 μL of virus containing buffer was injected into the lower G or L and 6 μL into those receiving virus both G+L. The small amount of solution remained in loco in the G or the L [27]. The needle was left inside the structures for one minute to prevent the leakage of the solution. All nerve fibers in the infiltrated area could pick the virus up. The appearance of virus in the TriG indicated the successful spreading of the virus.
Sympathectomy
Prior to the virus inoculation (2-4 days), SCG was removed from both sides. Animals exhibiting ptosis and myosis (signs of sympathectomy) were used for the inoculation. The success of sympathectomy was also confirmed by the abscence of GFP labeling in the IML. In this animal group we could separately examine the parasympathetic neuronal chain.
Preparing tissues for investigation
Six groups were included in the experiment. Numbers indicate the animals which survived.
Group 1.5 intact rats recieved virus in the lower G
Group 2.5 intact rats recieved virus in the lower L
Group 3.4 intact rats received virus into both G+L
Group 4.3 sympathectomized rats received virus in the lower G
Group 5.3 sympathectomized rats received virus in the lower L
Group 6.2 sympathectomized rats received virus in the lower G+L
The animals were sacrificed 96 h after inoculation. We had to use this survival period leaving enough time for replication of the small amount of virus. The animals were anesthetized by chloral hydrate (35 mg/100 gr bw), the animals were perfused with potassium phosphate buffer (KPB) (0.1 M, pH 7.4) containing 4% paraformaldehyde (Merck, Darmstadt, Germany) through the ascending aorta. The components of the buffer were purchased from Sigma-Aldrich (St. Louis, MO). The Submandibular Ganglion (SMG), SCG, upper thoracic part of the spinal cord was removed. Then the head was placed in the stereotaxic instrument, the skull was opened and the brain was transected 1.6 mm behind the bregma according to the Stereotaxic Atlas [28]. Then the brain behind the cut, TriG and OG were removed. The tissues were postfixed overnight, then from the forebrain only the hypothalamus was further processed. All tissues were washed in 0,1 M KPB and placed in sucrose solution (15%) (Reanal, Budapest, Hungary), then were embedded in Cryomatrix (Thermo Shandon, Pittsburg, PA). 20 μm thick sections were cut on cryostat (Cryotome, Thermo Shandon, Pittsburg, PA). The virus labeling (presence of GFP) was mapped and photographs were recorded by a multi-photon confocal microscope (Radiance 2100 Rainbow Multiphoton Imaging System, Bio-Rad Laboratories, USA, coupled to an Eclipse E800 microscope, Nikon, USA) using a Zen 2012 software.
Double labeling immunohistochemistry
One of the four series of the hypothalamic sections was stained for OT-neurophysin (OT-N), VP-neurophysin (VP-N), CCK, CRH or ORX immunoreactivity. After washing, the slides were treated with 1% Triton X-100 (Reanal) for better penetration of the antibody. OT-N and VP-N antisera were raised in mouse and characterized by Ben-Barak et al. [29] and were obtained as a kind gift from Dr. Gainer (NIH). Both were applied in a dilution of 1:200. CCK was raised in rabbit and characterized by Ciofi and Tramu [30]. CRH was purchased from Penninsula (San Carlos, CA, USA) and used in 1:500 dilutions. ORX antiserum was raised in goat, and purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA) and used in 1:500 dilutions. Sections of the brainstem exhibiting GFP labeling were stained for Dopamine β-Hydroxylase (DBH) (indicating the presence of noradrenaline), Vesicular Acethylcholine Transporter (VAChT), Somatostatin (SS), Serotonin (SER), Leu-Enkephalin (L-ENK), OT-N or ORX immunoreactivity. The upper three segments of the spinal cord were stained for VAChT, DBH, OT-N, VP-N, L-ENK, CRH and ORX immunoreactivities. Sections of the SCG were stained for DBH, NPY, VAChT or VIP immunoreactivity. OG and SMG were stained for VAChT, NPY, DBH or VIP immunoreactivity. VIP, SS, NPY, L-ENK and SER antisera were raised in rabbit by Görcs and characterized by Gulyás et al. [31] (VIP, SS), Borostyánkoi et al. (NPY) [32], Lopez Costa et al. [33] (L-ENK, SER) and used in a dilution of 1:500. VAChT antiserum was raised in guinea pig and purchased from EMD Millipore (Temecula, CA). It was used in a dilution of 1:500. In every case we used biotinylated second antibodies. In each case the final reaction product of immunostaining was visualized by streptavidin Cy3 conjugate (Sigma-Aldrich, St. Louis, MO).
Evaluation of the number of virus labeled neurons in the hypothalamus
From the hypothalamus, starting at P1, 6 mm behind the bregma, 4 parallel series of 20 μm thick frontal sections were prepared. In every series we had 16 sections (one from every 100 μm thick tissue). The sections were mounted on gelatinized slides. In 4-8 sections of the first series containing PVN and 12-16 sections containing Pf the number of virus labeled cells were counted. These ten sections were immunostained.
Evaluation of the number of virus labeled neurons containing OT-N and VP-N in the PVN and ORX in the Pf
When the double labeling was completed the number of double labeled cells was also counted.
Specificity tests
The neurotropic spreading and exclusively retrograde transportation of our virus strain were previously tested [25]. In the case of inoculation of the animals through the external jugular vein did not result labeling in the TriG. This indicates the exclusively neurotropic spreading. Inoculation of G and L resulted in labeling in the pseudounipolar neurons of the TriG but not in the second order neurons. This fact clearly shows that the virus could not spread in an anterograde manner. Two specificity tests were carried out by: 1) omitting the primary or secondary antibodies prevented the immunostaining, 2) positive control staining with the antibodies. Occurrence of OT-N, VP-N and CCK immunoreactivities in the hypothalamic PVN is well known for thirty-fourty years and ORX in the Pf for seventeen years [24,30,34-36]. The presence of neuropeptides and neurotransmitter synthetizing enzymes in other investigated organs including OG, SMG, IML 37, 20, SCG, LC, rVLM, RPa, Gi, ISN, PVN and PF are also well established [20,22,37-42].
Results
Virus labeling
When the virus was injected into the G or L of both intact and sympathectomized rats labeling always appeared in the TriG. This signal indicated the successful spreading of the virus as it was previously demonstrated [25].
Groups 1, 2 and 3. In intact animals labeling was always observed in the ipsilateral structures belonging to the peripheral autonomic nervous system: in the SCG (Figure 1J, 1M, 1P and 1T), the OG (Figure 2A, 2D and 2G) of the animals of Group 1 and additionally in the SMG (Figure 2M, 2P, 2T and 2Z) of the animals of Group 2 and 3. Labeling was also seen in the ipsilateral IML of the three upper segments of the spinal cord (Figure 1D), the LC (Figure 3A), the ventral part of the Gigantocellular Reticular Nucleus (Gi) (Figure 3J) which arches above the pyramid, salivatory nuclei, the rostral VLM (rVLM) (Figure 3D), the PVN (Figure 4A, 4D and 4G) and the Pf (Figure 1J) as it was described in our previous paper [25]. In the hypothalamus the Pf and the PVN were labeled at both right and left sides. In the PVN the labeled cells were present mainly in the magnocellular but labeled cells also occurred in the periventricular parvocellular subdivision. In the Pf the virus labeled cells arched above the fornix and on the medial side.
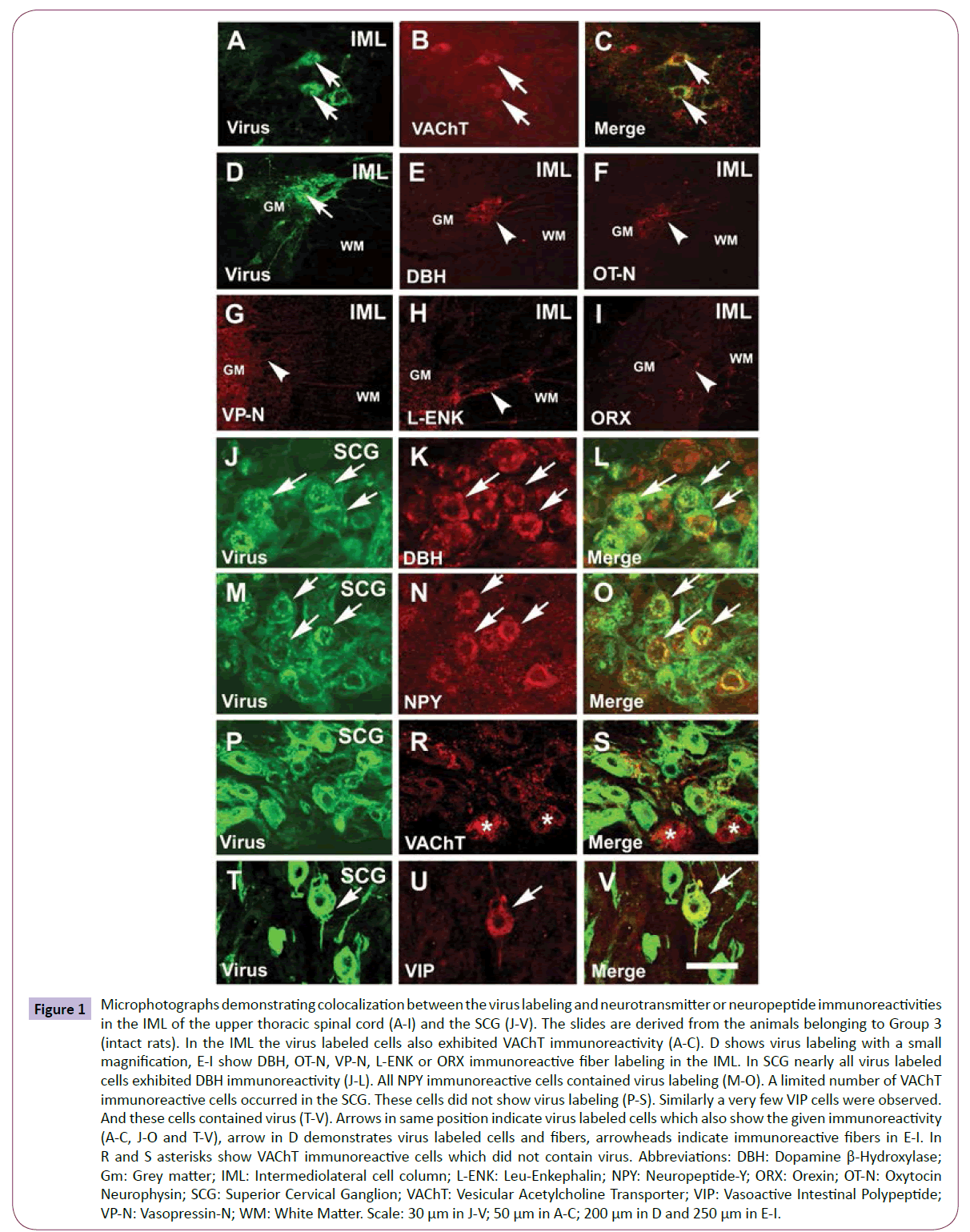
Figure 1: Microphotographs demonstrating colocalization between the virus labeling and neurotransmitter or neuropeptide immunoreactivities in the IML of the upper thoracic spinal cord (A-I) and the SCG (J-V). The slides are derived from the animals belonging to Group 3 (intact rats). In the IML the virus labeled cells also exhibited VAChT immunoreactivity (A-C). D shows virus labeling with a small magnification, E-I show DBH, OT-N, VP-N, L-ENK or ORX immunoreactive fiber labeling in the IML. In SCG nearly all virus labeled cells exhibited DBH immunoreactivity (J-L). All NPY immunoreactive cells contained virus labeling (M-O). A limited number of VAChT immunoreactive cells occurred in the SCG. These cells did not show virus labeling (P-S). Similarly a very few VIP cells were observed. And these cells contained virus (T-V). Arrows in same position indicate virus labeled cells which also show the given immunoreactivity (A-C, J-O and T-V), arrow in D demonstrates virus labeled cells and fibers, arrowheads indicate immunoreactive fibers in E-I. In R and S asterisks show VAChT immunoreactive cells which did not contain virus. Abbreviations: DBH: Dopamine β-Hydroxylase; Gm: Grey matter; IML: Intermediolateral cell column; L-ENK: Leu-Enkephalin; NPY: Neuropeptide-Y; ORX: Orexin; OT-N: Oxytocin Neurophysin; SCG: Superior Cervical Ganglion; VAChT: Vesicular Acetylcholine Transporter; VIP: Vasoactive Intestinal Polypeptide; VP-N: Vasopressin-N; WM: White Matter. Scale: 30 μm in J-V; 50 μm in A-C; 200 μm in D and 250 μm in E-I.
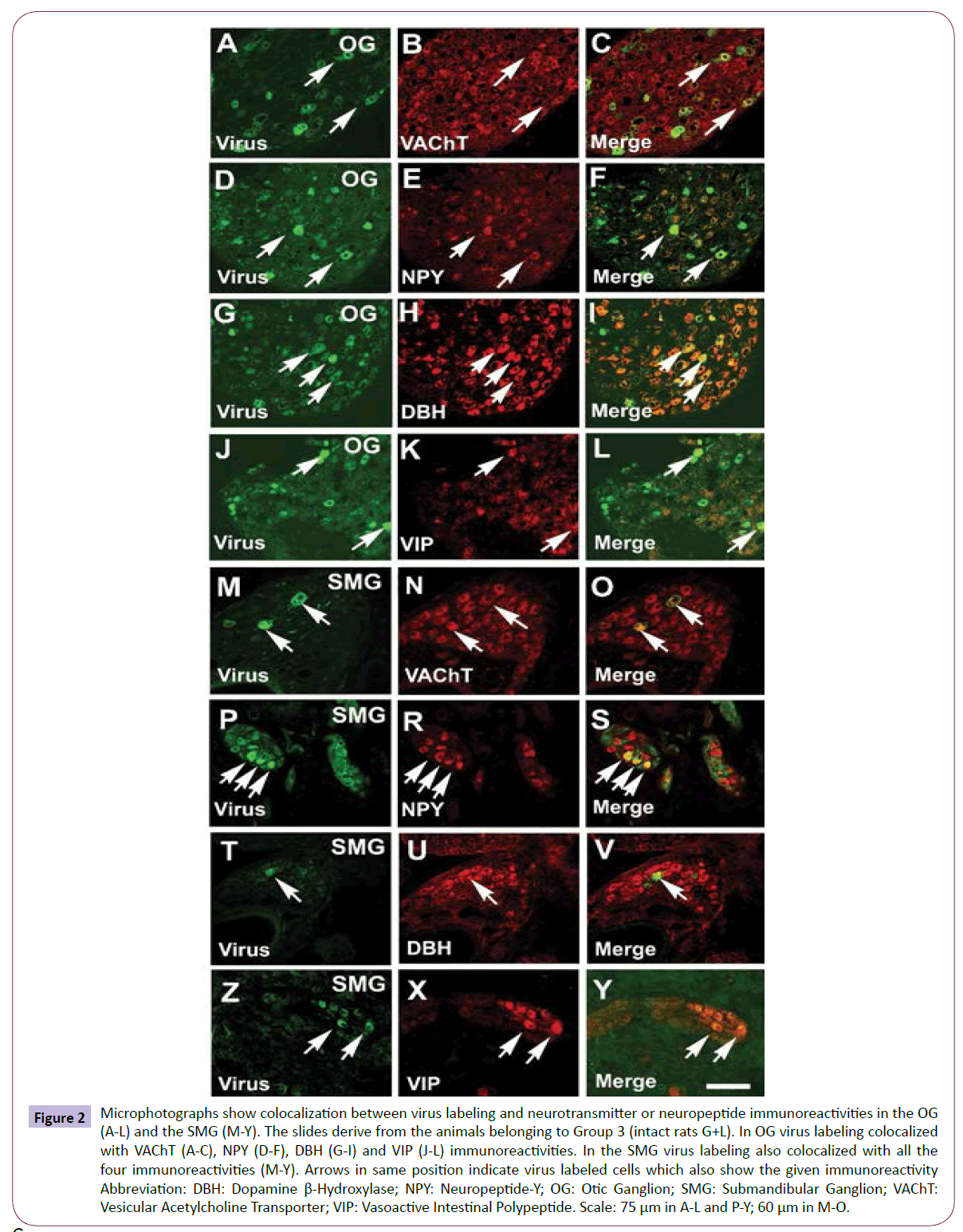
Figure 2: Microphotographs show colocalization between virus labeling and neurotransmitter or neuropeptide immunoreactivities in the OG (A-L) and the SMG (M-Y). The slides derive from the animals belonging to Group 3 (intact rats G+L). In OG virus labeling colocalized with VAChT (A-C), NPY (D-F), DBH (G-I) and VIP (J-L) immunoreactivities. In the SMG virus labeling also colocalized with all the four immunoreactivities (M-Y). Arrows in same position indicate virus labeled cells which also show the given immunoreactivity Abbreviation: DBH: Dopamine β-Hydroxylase; NPY: Neuropeptide-Y; OG: Otic Ganglion; SMG: Submandibular Ganglion; VAChT: Vesicular Acetylcholine Transporter; VIP: Vasoactive Intestinal Polypeptide. Scale: 75 μm in A-L and P-Y; 60 μm in M-O.
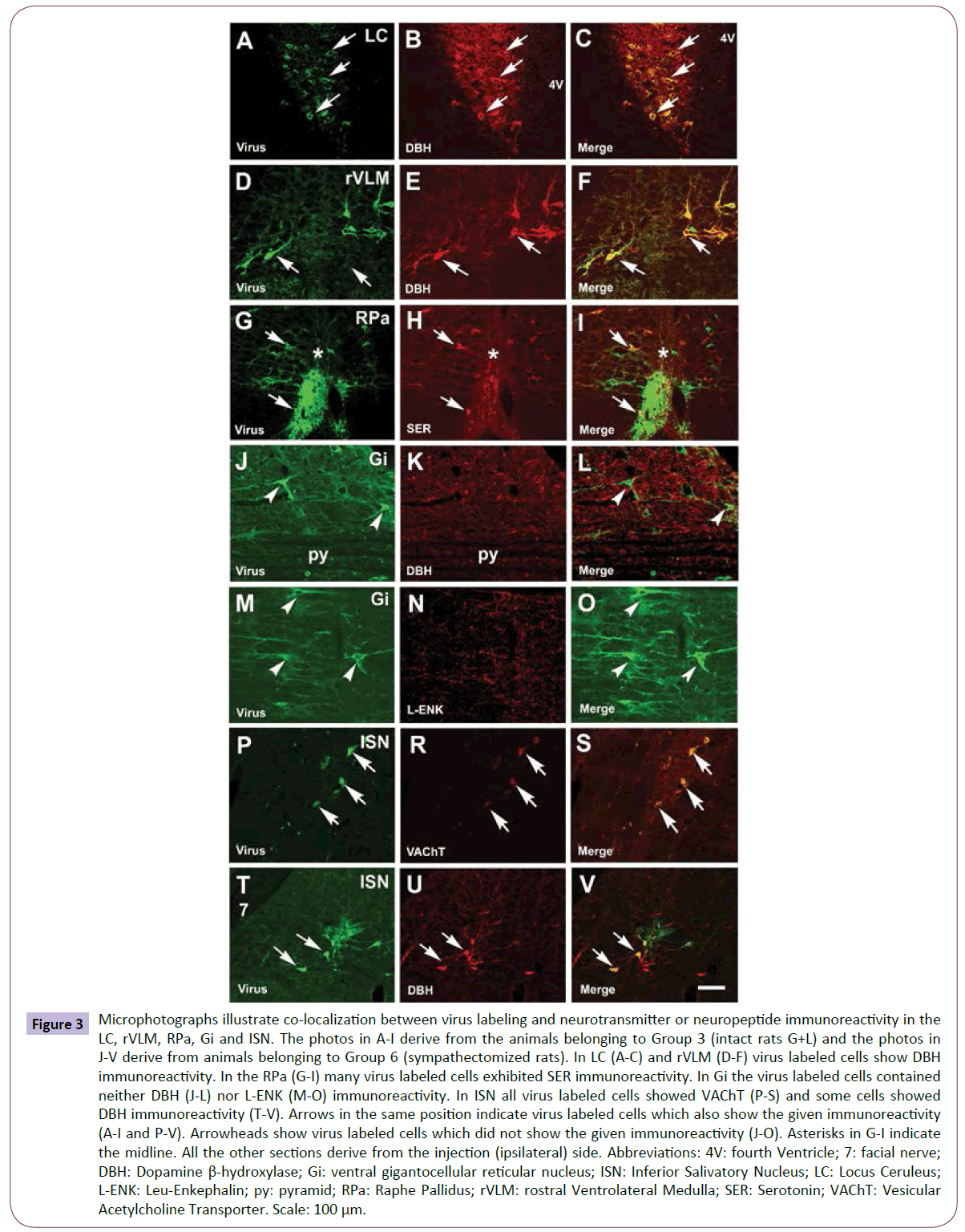
Figure 3: Microphotographs illustrate co-localization between virus labeling and neurotransmitter or neuropeptide immunoreactivity in the LC, rVLM, RPa, Gi and ISN. The photos in A-I derive from the animals belonging to Group 3 (intact rats G+L) and the photos in J-V derive from animals belonging to Group 6 (sympathectomized rats). In LC (A-C) and rVLM (D-F) virus labeled cells show DBH immunoreactivity. In the RPa (G-I) many virus labeled cells exhibited SER immunoreactivity. In Gi the virus labeled cells contained neither DBH (J-L) nor L-ENK (M-O) immunoreactivity. In ISN all virus labeled cells showed VAChT (P-S) and some cells showed DBH immunoreactivity (T-V). Arrows in the same position indicate virus labeled cells which also show the given immunoreactivity (A-I and P-V). Arrowheads show virus labeled cells which did not show the given immunoreactivity (J-O). Asterisks in G-I indicate the midline. All the other sections derive from the injection (ipsilateral) side. Abbreviations: 4V: fourth Ventricle; 7: facial nerve; DBH: Dopamine β-hydroxylase; Gi: ventral gigantocellular reticular nucleus; ISN: Inferior Salivatory Nucleus; LC: Locus Ceruleus; L-ENK: Leu-Enkephalin; py: pyramid; RPa: Raphe Pallidus; rVLM: rostral Ventrolateral Medulla; SER: Serotonin; VAChT: Vesicular Acetylcholine Transporter. Scale: 100 μm.
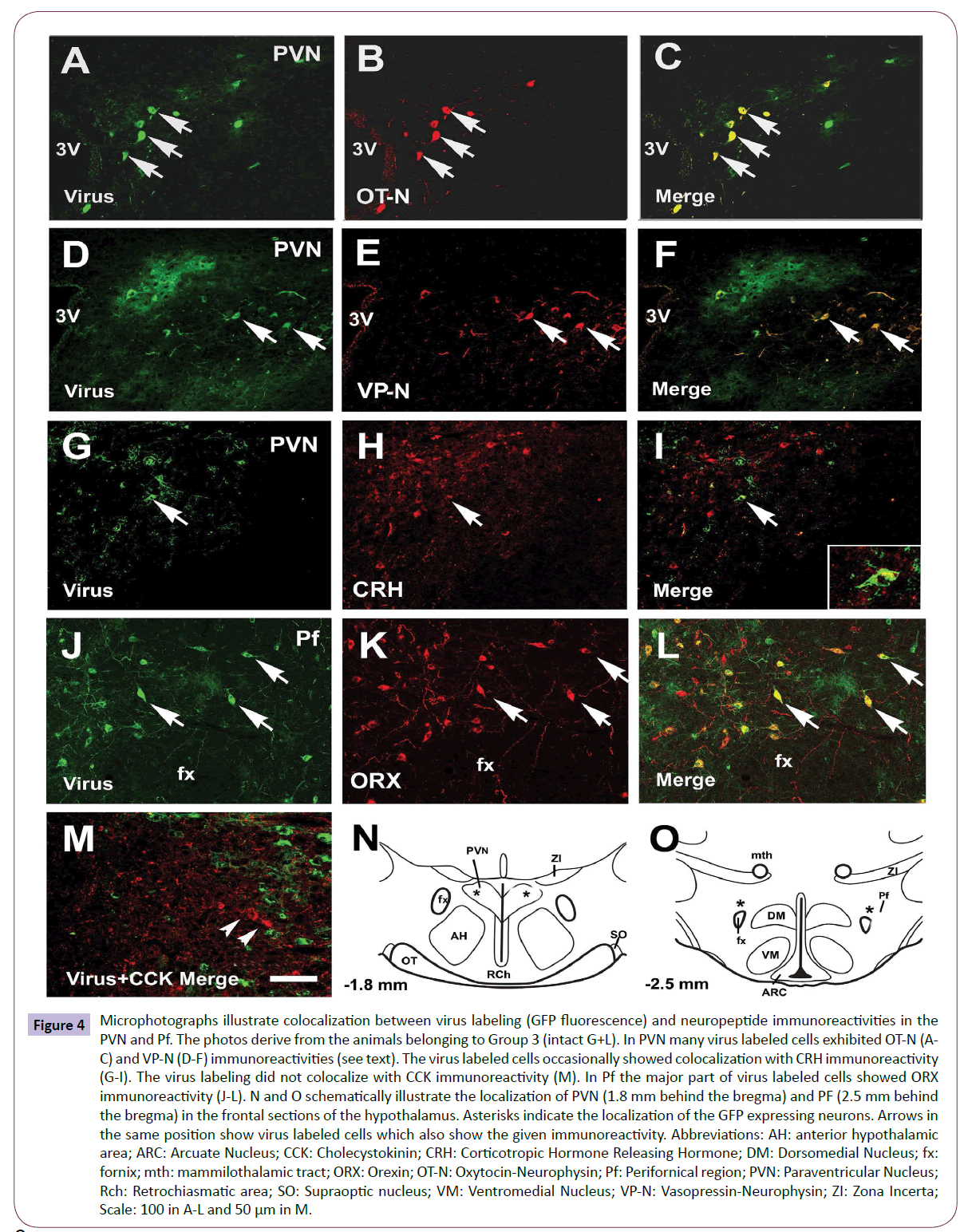
Figure 4: Microphotographs illustrate colocalization between virus labeling (GFP fluorescence) and neuropeptide immunoreactivities in the PVN and Pf. The photos derive from the animals belonging to Group 3 (intact G+L). In PVN many virus labeled cells exhibited OT-N (AC) and VP-N (D-F) immunoreactivities (see text). The virus labeled cells occasionally showed colocalization with CRH immunoreactivity (G-I). The virus labeling did not colocalize with CCK immunoreactivity (M). In Pf the major part of virus labeled cells showed ORX immunoreactivity (J-L). N and O schematically illustrate the localization of PVN (1.8 mm behind the bregma) and PF (2.5 mm behind the bregma) in the frontal sections of the hypothalamus. Asterisks indicate the localization of the GFP expressing neurons. Arrows in the same position show virus labeled cells which also show the given immunoreactivity. Abbreviations: AH: anterior hypothalamic area; ARC: Arcuate Nucleus; CCK: Cholecystokinin; CRH: Corticotropic Hormone Releasing Hormone; DM: Dorsomedial Nucleus; fx: fornix; mth: mammilothalamic tract; ORX: Orexin; OT-N: Oxytocin-Neurophysin; Pf: Perifornical region; PVN: Paraventricular Nucleus; Rch: Retrochiasmatic area; SO: Supraoptic nucleus; VM: Ventromedial Nucleus; VP-N: Vasopressin-Neurophysin; ZI: Zona Incerta; Scale: 100 in A-L and 50 μm in M.
Groups 4, 5 and 6. Only those sympathectomized rats were further examined where the labeling was missing in the IML. In the brainstem of these latter groups the labeling was only observed in the salivatory nuclei and the Gi. In the hypothalamus it was present in the PVN and the Pf, but the number of virus labeled cells was less than in intact rats. In sympathectomized animals the labeling further existed in the OG of the animals and additionally in the SMG of the animals of Groups 5 and 6.
Double labeling
The description of the descending autonomic pathways starts from the premotor third order hypothalamic neurons downwards to the periphery.
Third order (premotor) neurons in the hypothalamic nuclei: In the hypothalamic PVN of intact rats about half of the GFP labeled cells showed OT-N (Figures 4A-4C) or VP-N immunoreactivity (Figures 4D-4F). We have found only one virus labeled cell which also showed CRH immunoreactivity (Figures 4G-4I). The double labeled cell is showed by the insert in Figure 4I. CCK immunoreactivity did not colocalize with virus labeling (Figure 4M). In the Pf, the majority of virus labeled cells exhibited ORX immunoreactivity (Figures 4J-4L). Figures 4N and 4O schematically illustrate the localization of the virus labeled cells in the PVN and Pf.
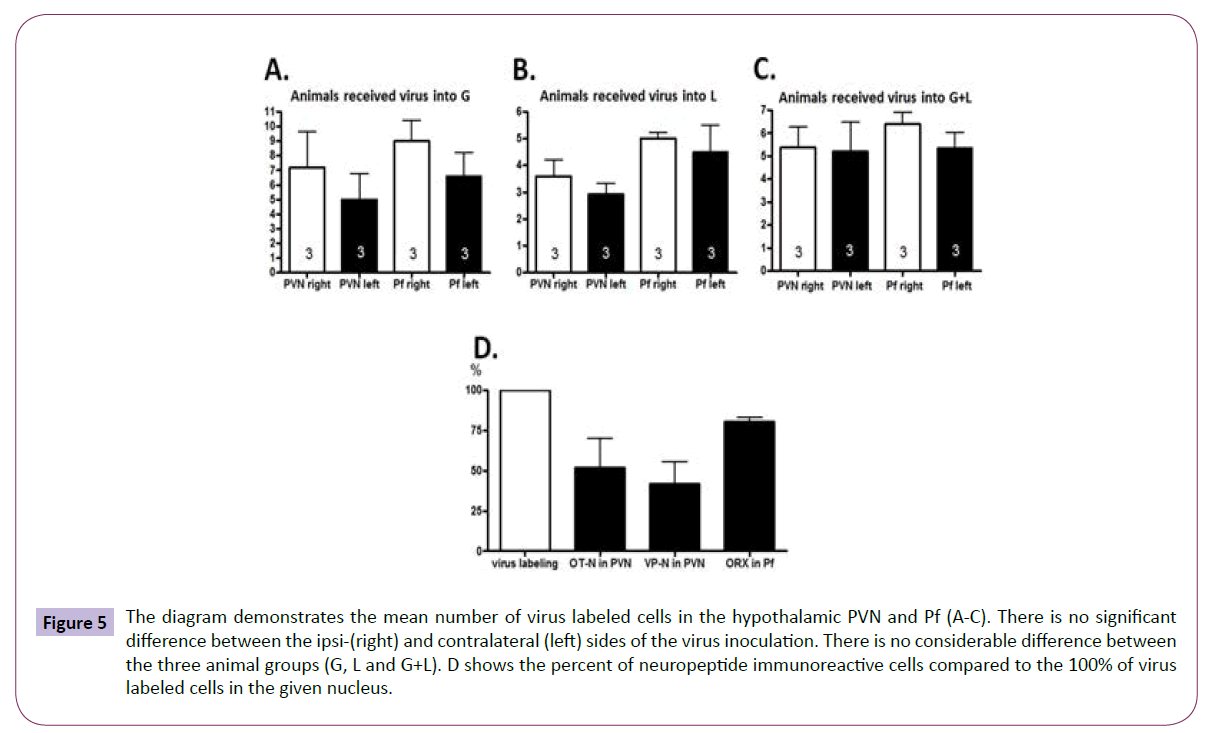
Quantitative analysis of the distributiob of virus labeled neurons and those containing OT-N and VP-N in the PVN and ORX in the Pf (Group 3): The number of the virus labeled cells was evaluated in the PVN and Pf at ipsi- and contralateral sides (Figures 5A-5C). Their number did not differ significantly from each other at the two sides of the PVN and Pf. Counting the cells revealed that about half of the virus labeled cells found in the PVN synthetized OT or VP (Figure 5D). Nearly all virus labeled cells in the Pf (Figure 5D) showed ORX immunoreactivity.
Figure 5: The diagram demonstrates the mean number of virus labeled cells in the hypothalamic PVN and Pf (A-C). There is no significant difference between the ipsi-(right) and contralateral (left) sides of the virus inoculation. There is no considerable difference between the three animal groups (G, L and G+L). D shows the percent of neuropeptide immunoreactive cells compared to the 100% of virus labeled cells in the given nucleus.
Third order (premotor) neurons in the brainstem (Groups 1-3, intact rats): In the LC and the rVLM the virus labeled neurons exhibited DBH immunoreactivity (Figures 3A-3F, respectively) and received ORX fibers (not shown). In the raphe pallidus (RPa) (Figures 3G- 3I) and in the raphe magnus (RMg) (not shown) the virus labeled cells were SER immunoreactive. OT-N fibers entered the VLM, but not the LC (not shown).
Second order (preganglionic) parasympathetic neurons in the brainstem of sympathectomized rats (Groups 4-6): The virus labeled Gi neurons arching above the pyramid did not exhibit DBH (Figures 3J-3L), L-ENK (Figures 3M-3O), SER, SS and VAChT immunoreactivities (not shown). However, in the neighborhood of virus labeled cells many fibers, immunoreactive for the above-mentioned transmitters and peptides, were observed (not shown). The Gi neurons are common command neurons for both sympathetic and parasympathetic outflows, as we demonstrated previously [25]. Second order neurons of the parasympathetic pathway located in the salivatory nuclei were studied in sympathectomized rats. The neurons in these nuclei were immunopositive for VAChT (Figures 3P-3S). Virus labeled cells rarely showed colocalization with DBH immunoreactivity as well (Figures 3T-3V).
Second order (preganglionic) sympathetic neurons (Groups 1-3, intact rats): In the IML of the upper three segments of the spinal cord the preganglionic virus containing cell bodies were desintegrated in many cases; however, when the cells were intact we found colocalization with VAChT immunoreactivity (Figures 1A-1C). The IML, where the virus labeling was always intensive in intact rats (Figure 1D), received many noradrenergic (Figure 1E), less oxytocinergic (Figure 1F), a few vasopressinergic (Figure 1G), enkephalinergic (Figure 1H), but not CRH immunoreactive fibers; however, the posterior horn showed dense CRH fiber labeling indicating that the antibody staining was successful (not shown). Orexin fibers were very sparse in the IML (Figure 1I).
First order (postganglionic) sympathetic neurons (Groups 1-3, intact rats): In the SCG many virus labeled cells were found. All virus labeled postganglionic cells contained DBH (Figures 1J-1L), and many of them, but not all contained NPY immunoreactivity (Figures 1M-1O). Some VAChT immunoreactive cells were also seen in the SCG. We have not found colocalization between the virus labeling and VAChT immunoreactivity; however, many VAChT immunoreactive fibers surrounded the virus labeled cells (Figures 1P-1S). Very rarely a few VIP cells were found and there was colocalization between virus labeling and VIP immunoreactivity as well (Figures 1T-1V).
First order (postganglionic) parasympathetic neurons (Groups 1-3 intact and Groups 4-6 sympathectomized rats): In the OG, some virus labeled cells was found. All labeled cells showed VAChT immunoreactivity (Figures 2A-2C) and about half of them were NPY immunoreactive (Figures 2D-2F). Many cells were DBH immunoreactive and nearly all viruses labeled cells showed colocalization with this immunoreactivity (Figures 2G-2I). VIP immunoreactive cells were also observed in the OG and the virus labeled cells partially colocalized with this immunoreactivity (Figures 2J-2L).
In the SMG the virus labeling also appeared. The virus labeled cells showed VAChT (Figures 2M-2O), NPY (Figures 2P-2S), DBH (Figures 2T-2V) and VIP immunoreactivities (Figures 2Y and 2Z).
Discussion
In our previous paper besides the demonstration of the descending autonomic pathway to the lower G and L we clearly demonstrated that not only the L but the G also received both sympathetic and parasympathetic innervation [25]. The parasympathetic fibers of the lower G derive from the OG, and the parasympathetic fibers of the lower L derive from both OG and SMG. We traced the members of the descending sympathetic and parasympathetic pathways step-by-step using transneuronal retrograde spreading virus. Both sympathetic and parasympathetic premotor (third order) neurons were located in the hypothalamus and the brainstem. The second order neurons of the parasympathetic pathway may be present in the brainstem and the sympathetic neurons in the spinal cord. It was also revealed that the neurons of the Gi located above the pyramid are involved in both sympathetic and parasympathetic descending pathways.
In the present work we further investigated these pathways. In spite of the fact that using Phaseolus vulgaris tracing technique, demonstrated that mainly the parvocellular cells of the PVN project to the intermediolateral cell column of the spinal cord, observed virus labeling in the magnocellular subdivisions as well after virus inoculation of the submandibular gland [34,43]. The PVN is devided into 3 magnocellular and 5 parvocellular subdivisions and in the present experiment the majority of the virus labeled cells were located in the lateral magnocellular subdivision [34,44]. The number of virus labeled neurons in the PVN and Pf of animals where the virus was injected in the G (Group 1) or in the L (Group 2) was nearly the same (Figures 5A and 5B) as in those received virus in both places (Group 3, Figure 5C). Whereas the number of virus labeled cells was much lower in sympathectomized rats (Groups 4-6).
In this present work we also showed that the hypothalamic premotor third order autonomic neurons located in the PVN use OT-N and VP-N (51% vs. 41% respectively), but not CCK and CRH. We have to consider that the virus injected animals did not receive colchicine, which is usually used to demonstrate CRH or CCK cell bodies in PVN. Because the colchicine prevents the axonal transportation we could not use this pretreatment. The virus labeled neurons located in the Pf use ORX (about 80%) for influencing autonomic responses of the lower G and L. It was demonstrated three years ago that a retrograde spreading virus injected in the mammary gland of lactating rats appeared in the PVN and the subpopulation of virus labeled neurons also showed OT-N immunoreactivity [20]. Geerling et al. [9] injected retrogradely transported virus in the suprarenal gland and the kidney. The labeling appeared in ORX immunoreactive neurons of the Pf. They supposed that the ORX neurons regulate general sympathetic functions. Our data suggest that other hypothalamic peptides such as OT and VP have similar general function.
The fact that the mean number of GFP labeled cells is nearly the same in the animals receiving virus either in the G or the L, as in those receiving virus in both places at the same time suggests, that the same PVN neurons participate in the innervation of G and L through a multisynaptic descending pathway. The presence of OT-N, VP-N and ORX immunoreactive fibers was demonstrated in the IML of the spinal cord where the preganglionic sympathetic neurons are located [45-48]. Oxitocinergic, vasopressinergic and orexinergic fibers are also found in the brainstem [49, 50]. PVN neurons densely innervate the parasympathetic preganglionic neurons located in the SSN and less in the ISN and some fibers descend to IML which are involved in the transynaptic innervation of the G and L [25,51,52].
The amount of data concerning the chemical coding of the hypothalamic parasympathetic command neurons which is involved in the descending innervation of lower G and L is limited. A recent paper by Hettigoda et al. [43], who described the central neurons which were involved in both sympathetic and parasympathetic regulation of the submandibular gland, did not examine the chemical nature of these neurons. They hypothesized that whilst the peripheral sympathetic and parasympathetic pathways were separate, the distribution of premotor neurons in higher brain regions often overlapped.
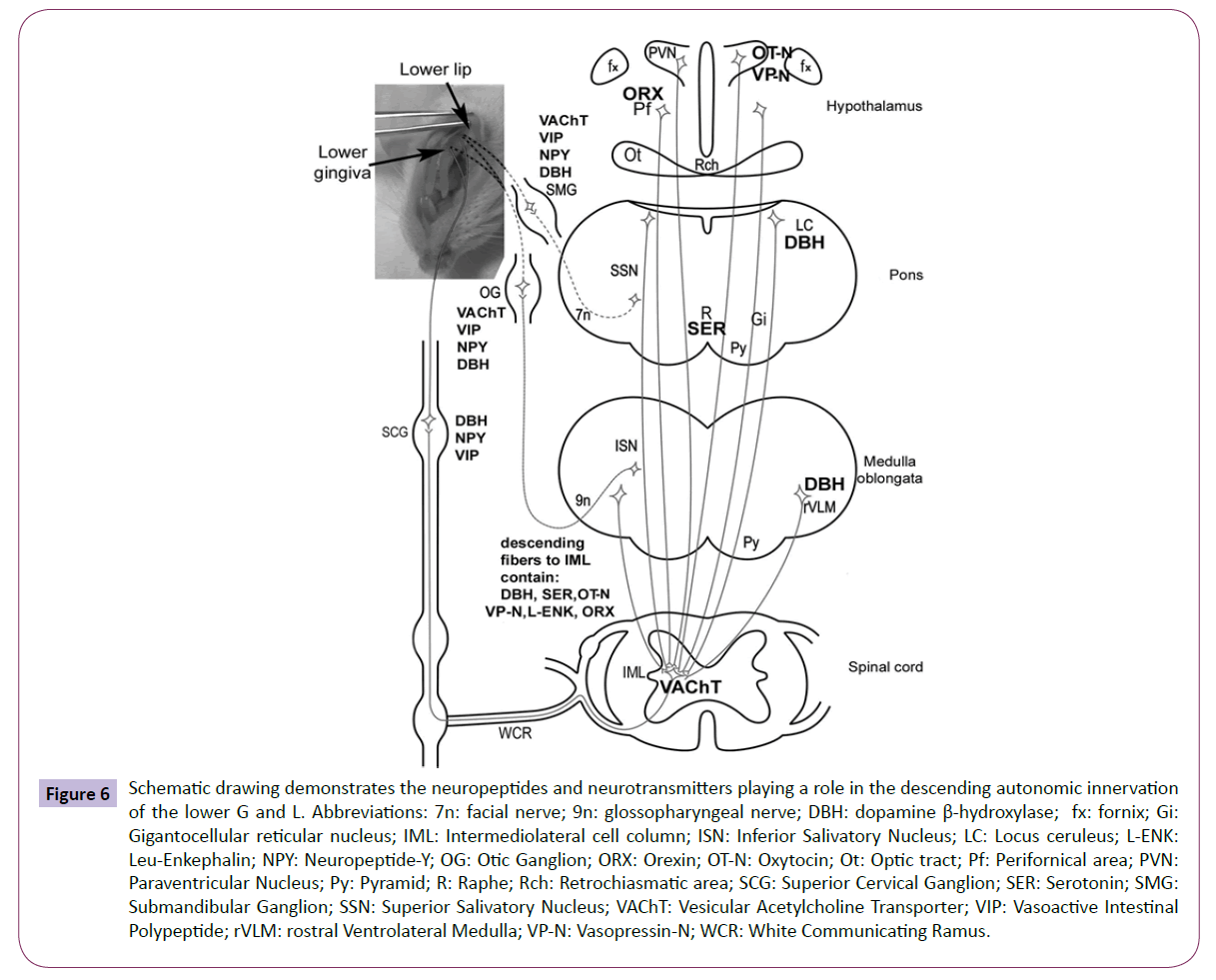
Our study on the characterization of the members of the descending autonomic pathways to the G and L summarizes partially the data available in the literature and what we have found. Figure 6 schematically illustrates the chemical coding of the entire length of the descending pathways. From PVN OT and VP fibers descend to the brainstem LC, VLM, ISN, SSN and GI. From LC and VLM noradrenergic fibers, and directly from PVN oxytocinergic and vasopressinergic fibers, descend to the IML. From the ISN, SSN cholinergic and from GI non-characterized fibers reach the otic ganglion, from the IML to the SCG also cholinerg fibers descend. In OG VAChT, VIP, NPY and DBH, in the SCG DBH, NPY and VIP immunoreactive neurons were found that may innervate G and L.
Figure 6: Schematic drawing demonstrates the neuropeptides and neurotransmitters playing a role in the descending autonomic innervation of the lower G and L. Abbreviations: 7n: facial nerve; 9n: glossopharyngeal nerve; DBH: dopamine β-hydroxylase; fx: fornix; Gi: Gigantocellular reticular nucleus; IML: Intermediolateral cell column; ISN: Inferior Salivatory Nucleus; LC: Locus ceruleus; L-ENK: Leu-Enkephalin; NPY: Neuropeptide-Y; OG: Otic Ganglion; ORX: Orexin; OT-N: Oxytocin; Ot: Optic tract; Pf: Perifornical area; PVN: Paraventricular Nucleus; Py: Pyramid; R: Raphe; Rch: Retrochiasmatic area; SCG: Superior Cervical Ganglion; SER: Serotonin; SMG: Submandibular Ganglion; SSN: Superior Salivatory Nucleus; VAChT: Vesicular Acetylcholine Transporter; VIP: Vasoactive Intestinal Polypeptide; rVLM: rostral Ventrolateral Medulla; VP-N: Vasopressin-N; WCR: White Communicating Ramus.
It was demonstrated that besides the well known neurotransmitter and neuropeptides the parasympathetic OG and SMG cell bodies also contain DBH which is a charateristic sympathetic neurotransmitter converting enzyme. Hoard and her co-workers [53] found that the half of the cholinergic neurons of human and primate intrinsic parasympathetic cardiac ganglia have a dual cholinergic/noradrenergic phenotype. Another research group found DBH in the OG. They supposed that this enzyme is an embryological remnant and it has no functional significance [37]. The question arises whether it is true for the oral structures as well.
Acknowledgement
We are very grateful to Anna Takáts for her excellent technical assistance. This work was supported by the Department of Human Morphology and Developmental Biology, Semmelweis University and the Department of Conservative Dentistry and Endodontics, Faculty of Dentistry, Semmelweis University, Budapest, Hungary, Swiss-Hungarian Cooperation Programme grant (SH/7/2/8) to Zsolt Boldogkoi and OTKA-K grant No 112364 to Zsolt Lohinai. All co-authors have agreed to the publication of the paper and they have reported no potential conflicts.
References
- Enquist LW (2002) Exploiting circuit-specific spread of pseudorabies virus in the central nervous system: Insights to pathogenesis and circuit tracers. J Infect Dis 186 Suppl 2: S209-214.
- Izumi H, Karita K (1991) Vasodilator responses following intracranial stimulation of the trigeminal, facial and glossopharyngeal nerves in the cat gingiva. Brain Res 560: 71-75.
- Lundy FT, Linden GJ (2004) Neuropeptides and neurogenic mechanisms in oral and periodontal inflammation.Crit Rev Oral Biol Med 15: 82-98.
- Urbanovich VI, Vylegzhanina TA, Maneeva OA, Kuznetsova TE, Ryzhkovskaya EL (1999) Adrenergic innervation of the gingiva in experimental periodontitis. BullExpBiolMed127: 564-568.
- Kuchiiwa S, Kuchiiwa T (1996) Autonomic and sensory innervation of cat molar gland and blood vessels in the lower lip, gingiva and cheek. J AutonNervSyst 61: 227-234.
- Fehér E, Zelles T, Nagy G (1999) Immunocytochemicallocalisation of neuropeptide-containing nerve fibres in human labial glands. Arch Oral Biol 44 Suppl 1: S33-37.
- Lohinai Z, Székely AD, Benedek P, Csillag A (1997) Nitric oxide synthase containing nerves in the cat and dog dental pulp and gingiva. NeurosciLett 227: 91-94.
- Strack AM, Sawyer WB, Platt KB, Loewy AD (1989) CNS cell groups regulating the sympathetic outflow to adrenal gland as revealed by transneuronal cell body labeling with pseudorabies virus.Brain Res 491: 274-96.
- Geerling JC, Mettenleiter TC, Loewy AD (2003) Orexin neurons project to diverse sympathetic outflow systems. Neuroscience 122: 541-550.
- Tóth IE, Banczerowski P, Boldogkoi Z, Tóth JS, Szabó A, et al. (2008) Cerebral neurons involved in the innervation of both the adrenal gland and the ovary: a double viral tracing study. Brain Res Bull 77: 306-311.
- Li YW, Ding ZQ, Wesselingh SL, Blessing WW (1992) Renal and adrenal sympathetic preganglionic neurons in rabbit spinal cord: tracing with herpes simplex virus. Brain Res 573: 147-152.
- Schramm LP, Strack AM, Platt KB, Loewy AD (1993) Peripheral and central pathways regulating the kidney: a study using pseudorabies virus. Brain Res 616: 251-262.
- Reichart A, BoldogkoiZs, Lenkei Z, Medveczky I, Palkovits M (2000) Neurochemical characterization of kidney regulating brainstem neurons identified by pseudorabiestransneuronal labeling. Neurobiology 8: 277-80.
- Vizzard MA, Brisson M, de Groat WC (2000) Transneuronal labeling of neurons in the adult rat central nervous system following inoculation of pseudorabies virus into the colon. Cell Tissue Res 299: 9-26.
- Marson L, Carson 3rd CC (1999) Central Nervous System Innervation of the Penis, Prostate, and Perineal Muscles: A Transneuronal Tracing Study. MolUrol 3: 43-50.
- Rouzade-Dominguez ML, Miselis R, Valentino RJ (2003) Central representation of bladder and colon revealed by dual transsynaptic tracing in the rat: substrates for pelvic visceral coordination. Eur J Neurosci 18: 3311-3324.
- Jansen ASP, Ter Horst GJ, Mettenleiter TC, Loewy AD(1992) CNS cell groups projecting to the submandibular parasympathetic preganglionic neurons in the rat: a retrograde transneuronal viral cell body labeling study. Brain Res 572: 253-260.
- Rezek O, Boldogkoi Z, Tombácz D, Kovágó C, Gerendai I, et al. (2008) Location of parotid preganglionic neurons in the inferior salivatory nucleus and their relation to the superior salivatory nucleus of rat. NeurosciLett 440: 265-269.
- Gerendai I, Tóth IE, Kocsis K, Boldogkoi Z, Medveczky I, et al. (2001) Transneuronallabelling of nerve cells in the CNS of female rat from the mammary gland by viral tracing technique. Neuroscience 108: 103-118.
- Köves K, Györgyi Z, Szabó FK, Boldogkői Z (2012) Characterization of the autonomic innervation of mammary gland in lactating rats studied by retrograde transynaptic virus labeling and immunohistochemistry. ActaPhysiol Hung 99: 148-158.
- Strack AM, Sawyer WB, Hughes JH, Platt KB, Loewy AD (1989) A general pattern of CNS innervation of the sympathetic outflow demonstrated by transneuronalpseudorabies viral infections. Brain Res 491: 156-162.
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, et al. (1998)Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92: 573-85.
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, et al. (1998) The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. ProcNatlAcadSci U S A 95: 322-327.
- Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, et al. (1999) Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. ProcNatlAcadSci U S A 96: 748-753.
- Szabó E, BoldogkoiZs, Csáki Á, TóthZs, Köves K (2015)Identification of autonomic neuronal chains innervating gingiva and lip. Autonomic Neuroscience: Basic and Clinical 190: 10-19.
- Boldogköi Z, Erdélyi F, Fodor I (2000) A putative latency promoter/enhancer (P(LAT2)) region of pseudorabies virus contains a virulence determinant. J Gen Virol 81: 415-420.
- BoldogkoiZs, Reichart A, Tóth IE, Sik A, Erdélyi F, et al. (2002) Construction of recombinant pseudorabies viruses optimized for labeling and neurochemical characterization of neural circuitry. Brain Re Mol Brain Res 109: 105-118.
- Fenik VB, Kubin L (2009) Differential localization of carbachol- and bicuculline-sensitive pontine sites for eliciting REM sleep-like effects in anesthetized rats. J Sleep Res 18: 99-112.
- Ben-Barak Y, Russel JT, Whitnall MH, Ozato K, Gainer H (1985) Neurophysin in the hypothalamo-hypophysial system, I. Production and characterization of monoclonal antibodies. J Neurosci 5: 81-97.
- Ciofi P, Tramu G (1990) Distribution of cholecystokinin-like-immunoreactive neurons in the guinea pig forebrain. J Comp Neurol 300: 82-112.
- Gulyás AI, Görcs TJ, Freund TF (1990) Innervation of different peptide-containing neurons in the hippocampus by GABAergicseptal afferents. Neuroscience 37: 31-44.
- Borostyánkoi ZA, Görcs TJ, Hámori J (1999) Immunocytochemical mapping of NPY and VIP neuronal elements in the cat subcortical visual nuclei, with special reference to the pretectum and accessory optic system. AnatEmbryol (Berl) 200: 495-508.
- López Costa JJ, Marter S, Görcs TJ, Saavedra JP, Priestley JV (1991) Enkephalin and serotonin innervation of somatostatin-immunoreactive medullary reticular formation neurones. Brain Res 548: 300-304.
- Swanson LW, Kuypers HGJM (1980)Theparaventricular nucleus of the hypothalamus: Cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurology 194: 555-570.
- Zimmerman EA, Robinson AG, Husain MK, Acosta M, Frantz AG, et al. (1974) Neurohypophysial peptides in the bovine hypothalamus: the relationship of neurophysin I to oxytocin, and neurophysin II to vasopressin in supraoptic and paraventricular regions. Endocrinology 95: 931-936.
- Kiss JZ, Williams TH, Palkovits M (1984) Distribution and projections of cholecystokinin-immunoreactive neurons in the hypothalamic paraventricular nucleus of rat. J Comp Neurol 227: 173-181.
- Hardebo JE, Suzuki N, Ekblad E, Owman C (1992) Vasoactive intestinal polypeptide and acetylcholine coexist with neuropeptide Y, dopamine-beta-hydroxylase, tyrosine hydroxylase, substance P or calcitonin gene-related peptide in neuronal subpopulations in cranial parasympathetic ganglia of rat. Cell Tissue Res 267: 291-300.
- Wojtkiewicz J, Juranek JK, Kowalski I, Bladowski M, Calka J et al. (2011) Immunohistochemical characterization of superior cervical ganglion neurons supplying porcine parotid salivary gland. NeurosciLett 500: 57-62.
- Schwarz LA, Luo L (2015) Organization of the locus coeruleus-norepinephrine system. CurrBiol 25: R1051-1056.
- Xu ZQ, Hökfelt T (1997) Expression of galanin and nitric oxide synthase in subpopulations of serotonin neurons of the rat dorsal raphe nucleus. J ChemNeuroanat 13: 169-187.
- Hökfelt T, Millhorn D, Seroogy K, Tsuruo Y, Ceccatelli S, et al. (1987) Coexistence of peptides with classical neurotransmitters. Experientia 43: 768-780.
- Choy VJ, Watkins WB (1977) Immunocytochemical study of the hypothalamo-neurohypophysial system. II. Distribution of neurophysin, vasopressin and oxytocin in the normal and osmotically stimulated rat. Cell Tissue Res 180: 467-490.
- Hettigoda NS, Fong AY, Badoer E, McKinley MJ, Oldfield BJ, et al. (2015) Identification of CNS neurons with polysynaptic connections to both the sympathetic and parasympathetic innervation of the submandibular gland. Brain StructFunct 220: 2103-2120.
- Kiss JZ, Martos J, Palkovits M (1991) Hypothalamic paraventricular nucleus: a quantitative analysis of cytoarchitectonic subdivisions in the rat. J Comp Neurol 313: 563-573.
- Jójárt J, Jójárt I, Boda K, Gálfi M, Mihály A, et al. (2009) Distribution of oxytocin-immunoreactive neuronal elements in the rat spinal cord. ActaBiol Hung 60: 333-346.
- Buijs RM (1978) Intra- and extrahypothalamic vasopressin and oxytocin pathways in the rat. Path ways to the limbic system, medulla oblongata and spinal cord. Cell Tissue Res 192: 423-435.
- Nilaver G, Zimmerman EA, Wilkins J, Michaels J, Hoffman D, et al. (1980) Magnocellular hypothalamic projections to the lower brain stem and spinal cord of the rat. Immunocytochemical evidence for predominance of the oxytocin-neurophysin system compared to the vasopressin-neurophysin system. Neuroendocrinology 30: 150-8.
- Llewellyn-Smith IJ, Martin CL, Marcus JN, Yanagisawa M, Minson JB, et al. (2003) Orexin-immunoreactive inputs to rat sympathetic preganglionic neurons. NeurosciLett 351: 115-119.
- Lee SK, Ryu PD, Lee SY (2013) Differential distributions of neuropeptides in hypothalamic paraventricular nucleus neurons projecting to the rostral ventrolateral medulla in the rat. NeurosciLett 556: 160-165.
- Puskás N, Papp RS, Gallatz K, Palkovits M (2010) Interactions between orexin-immunoreactive fibers and adrenaline or noradrenaline-expressing neurons of the lower brainstem in rats and mice. Peptides 31: 1589-1597.
- Geerling JC, Shin JW, Chimenti PC, Loewy AD (2010) Paraventricular hypothalamic nucleus: axonal projections to the brainstem. J Comp Neurol 518: 1460-1499.
- Novak P (2007) Central anatomic network,In: Neurobiology of disease (Ed. Gilman S.) Elsevier Academic Press, Burlington, San Diego, London.
- Hoard JL, Hoover DB, Mabe AM, Blakely RD, Feng N, et al. (2008) Cholinergic neurons of mouse intrinsic cardiac ganglia contain noradrenergic enzymes, norepinephrine transporters, and the neurotrophin receptors tropomyosin-related kinase A and p75. Neuroscience 156: 129-142.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences