Effects of Ovariectomy and Estrogen Replacement on Expression of Brain Vasotocin Receptor Subtype Genes in the Catfish Heteropneustes fossilis
Rawat A, Chaube R and Joy KP
Rawat A1, Chaube R1 and Joy KP2*
1Department of Zoology, Centre of Advanced Study, Banaras Hindu University, Varanasi-221005, India
2Department of Biotechnology, Cochin University of Science and Technology, Kochi, India
- *Corresponding Author:
- Joy KP
Department of Biotechnology
Cochin University of Science and Technology
Kochi-682022, India
Tel: 918281274891
E-mail: kpjoybhu@gmail.com
Received date: January 25, 2016; Accepted date: February 23, 2016; Published date: February 27, 2016
Citation: Rawat A,Chaube R and Joy KP (2016) Effects of Ovariectomy and Estrogen Replacement on Expression of Brain Vasotocin Receptor Subtype Genes in the Catfish Heteropneustes fossilis. J Transl Neurosci. 2016, 1:1.
Copyright: © 2016, Rawat A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Ovariectomy and estradiol-17β (E2) replacement are classical approaches to study E2 feedback relationships on the brain-pituitary-neuroendocrine axis. Earlier studies in the catfish have shown that ovariectomy decreased brain and plasma vasotocin (VT) levels; a low dose of E2 replacement restored, but high doses further inhibited the VT levels. The feedback effects of E2 on VT secretion were similar to that on gonadotropin secretion. Previously, we have studied the expression of VT receptor gene subtypes (v1a1, v1a2 and v2a) in the brain, which showed seasonal sex dimorphic variations. But studies on the effect of E2 on VT receptor subtype gene expression are lacking in teleosts. We used ovariectomized-E2 replaced catfish model to demonstrate E2 effects on brain VT receptor gene expression. The experiment was conducted in the gonad active phase (prespawning phase). Ovariectomy (Ovx) for 3 weeks decreased the expression of v1a1 and v1a2 but not of v2a. A low dose of E2 (0.1 μg/g BW) replacement reversed the effect of ovariectomy and restored the expression of v1a1 and v1a2. A high dose of E2 (0.5 μg/g BW) replacement did not alter the transcript level of v1a1 compared to the Ovx-vehicle group but increased the v1a2 expression compared to the Ovx control group, lower than that of the sham control group. But the low and high doses of E2 replacement did not alter the v2a receptor transcript level. In conclusion, E2 modulates only the expression of V1a type receptor gene paralogs. VT may elicit the estrogen-dependent reproductive and behavioral effects through the V1a type receptors.
Keywords
Catfish; Ovariectomy; E2 replacement; Brain VT receptor subtypes; Gene expression
Introduction
Neurohypophyseal nonapeptide hormones (vasopressin- VP, oxytocin OT and vasotocin- VT) perform multiple regulatory functions related to osmoregulation, metabolism, stress, cardiovascular activity, circadian rhythm, reproduction and behavior in different vertebrates [1-6]. These effects are mediated through membrane-bound G-protein coupled receptors. Previously, three types of VP / VT receptors namely V1a, V1b and V2 subtypes have been reported in mammals, birds, amphibians and teleosts [7-12]. Oxytocin / Mestocin acts through a single receptor type (OTR / MTR), which in teleosts have two isoforms, Isotocin receptor- type 1 and 2 (ITR1 and ITR2) [13-16].
Though gonadal steroid hormones have been reported to modulate the secretion of the neurohypophysial nonapeptides in vertebrates, there are few studies that report the effect of steroid hormones on nonapeptide receptor activity in sub mammalian vertebrates [14, 17-29]. Such investigations are almost non-existent among teleosts. It was shown that gonadectomy reduced the concentrations of putative receptors for VT in the brain of amphibians [7]. A possible reason for limited studies may be the non-availability of sensitive assay methods. Rawat et al. [30] cloned and characterized three VT receptor genes in the catfish: two V1a type and one V2 type. This has enabled us to make RNA probes for the analysis of receptor gene expression in various tissues.
The catfish Heteropneustes fossilis has been extensively used to study the reproductive role of VT [21]. Apart from brain, VT is also synthesized in the ovary and the VT levels in brain, plasma and ovary were shown to vary according to the reproductive phases [31]. Subsequently, VT was demonstrated to influence ovarian steroidogenesis, oocyte maturation, oocyte hydration, prostaglandin synthesis and ovulation [32-35]. Steroid hormones including estradiol-17β (E2) were shown to alter the VT levels [25, 26]. E2 is the principal estrogen in teleosts and induces vitellogenin synthesis in the liver and growth of oocytes. Ovariectomy and E2 replacement are conventionally used to study the feedback effect of E2 on the brain-pituitary- neuroendocrine axis [36, 37].
The objective of the present study was to demonstrate estrogen regulation of expression of VT receptor sub type genes. For this, we employed ovariectomy and E2 replacement strategy, which is established for the catfish previously. The data show differential effects of the treatments on VT receptor gene expression, which has been reported apparently for the first time in this study.
Materials and Methods
Animal collection and acclimatization
Adult female Heteropneustes fossilis (50-55 g) were collected from local fish markets in Varanasi in the second week of May, which was late vitellogenic phase or prespawning phase: (9.05 ± 0.1% gonado-somatic index, GSI). They were maintained in the laboratory for a week under natural photoperiod (13.0 L: 11.0 D) and temperature (25 ± 2°C) to overcome stress due to transportation and fed daily with goat liver ad libitum. All experiments were performed in accordance with the guidelines of the Animal Ethics Committee, Banaras Hindu University, Varanasi.
Chemicals and reagents
Guanidine thiocyanate-phenol solution (Qiagen), Revert-Aid H Minus first strand cDNA synthesis kit (Fermentas), veriquest SYBR green qPCR master mix (Affymetrix) and DNase I (Ambion) were purchased through local suppliers. Agarose, tris base, glacial acetic acid, EDTA-Na2, proteinase K and other chemicals were of molecular grade and purchased from EMerck, Mumbai, India. The primers used were synthesized by Integrated DNA Technology (IDT), India. Diethyl pyrocarbonate (DEPC), MS-222 and estradiol-17β (E2) were purchased from Sigma Chemical Company, St. Louis, USA.
Experiments
Ovariectomy and E2 replacement
After acclimatization, five female fish were sacrificed and the brains collected in RNA later to make an initial control (IC) group. The operations were conducted according to the procedure of Senthilkumaran and Joy [36]. Female catfish were anesthetized by spraying 0.01% of MS-222 over the gills. A 4 cm long midventral incision was made anterior to the urogenital pore to expose the paired ovary. The ovaries were carefully detached from the peritoneal covering and removed. The cut end of the oviduct was cauterized with a hot needle to prevent regeneration and the incision sutured. The fish were treated with benzanthine penicillin (16000 IU/I) for 3-5 days to prevent skin infection. Thirty anesthetized fish were ovariectomized (ovx) and an equal number was sham operated and maintained for 3 weeks. At the end of the three weeks, 5 fish each from the ovx and sham operated groups were killed by decapitation and brains were collected in RNA later (ovx and sham ovx groups). For estrogen replacement, the 3-week ovx and sham operated fish were administered intraperitoneally with vehicle, 0.1 and 0.5 μg/g body weight (BW) E2 for three consecutive days, making the groups of ovx + vehicle, ovx + 0.1 μg/g BW E2, ovx + 0.5 μg/g BW E2, sham+ vehicle, sham + 0.1 μg/g BW E2 and sham + 0.5 μg/g BW E2. E2 was dissolved in a small volume of ethanol and then diluted with propylene glycol to prepare a stock solution. At the end of the three days, the fish were sacrificed by decapitation and the brains were collected in RNA later. Tissues in RNA later were stored in -20°C until further processing.
qPCR assay for VT receptor gene expression
Total RNA was extracted from the tissues (100 mg) stored in RNA later by a single step method of RNA isolation. RNA purity was checked by calculating A260 / A280 ratio. Samples having a ratio above 2.0 were only used. Absence of genomic DNA contamination in the RNA preparation was confirmed by using non-reverse transcribed samples as templates. In addition, the absence of DNA in total RNA was ensured by treating with DNAse I before proceeding for the first strand cDNA synthesis. Five μg of total RNA was reverse transcribed using random hexamer primers and Revert Aid M-MuLV reverse transcriptase in a 20 μL reaction volume (first strand cDNA synthesis kit, Fermantas), using the manufacturer’s protocol. Gene-specific primers were designed for v1a1, v1a2 and v2a from the respective sequences (Table 1). Primers for β-actin were used for the internal control. The specificity of each primer pair was confirmed by dissociation curve analysis. The qPCR assays were performed in triplicate for different samples using the specific primers and VeriQuest TM SYBR Green qPCR master mix with ROX (Affymetrix, Inc. Cleveland, Ohio USA) in a ABI Prism 7500 thermal cycler (Applied Biosystems, Foster, CA, USA) at 95°C (15 s), 60°C (1 min) for 40 cycles.
| Adaptation | Sequence (5’-3’) | ||
|---|---|---|---|
| qPCR primer | v1a1 | Forward | CCAAACTCCGCACCGTCAA |
| Reverse | ATGCGGATAGGGTCACTGCT (150 bp) | ||
| v1a2 | Forward | TAGTGTGCTGGGCACCGTT | |
| Reverse | GATCCAGGGGTTGCAGCAG(140 bp) | ||
| v2a | Forward | CAGCGTGAGCACCATCTCC | |
| Reverse | ATGCGGATAGGGTCACTGCT (173 bp) | ||
| DNA Control | β-actin | Forward | TGGCCGTGACCTGACTGAC |
| Reverse | CCTGCTCAAAGTCAAGAGCGAC(157 bp) |
Table 1: Primer used for quantitative PCR.
Each sample was run in a final volume of 20 μL containing 1 μL of cDNA, 10 pM of each primer, and 10 μL of SYBR Green PCR master mix. Specificity of amplicons was verified by melting curve analysis (60 to 95°C) after 40 PCR cycles.
As controls, the assays were performed without templates. No amplification was observed in the control studies. Cycle threshold (Ct) values were obtained from the exponential phase of the PCR amplification and target gene (v1a1, v1a2 and v2a) expression was normalized against β-actin gene expression to generate a Ct value. Comparative Ct (ΔΔCT) was used to quantify the target gene abundance [38].
Statistical analysis
The data were expressed as mean ± SEM of five replicates. Statistical analysis was carried out using two way ANOVA (p < 0.001), followed by Tukey’s test (p < 0.05).
Results
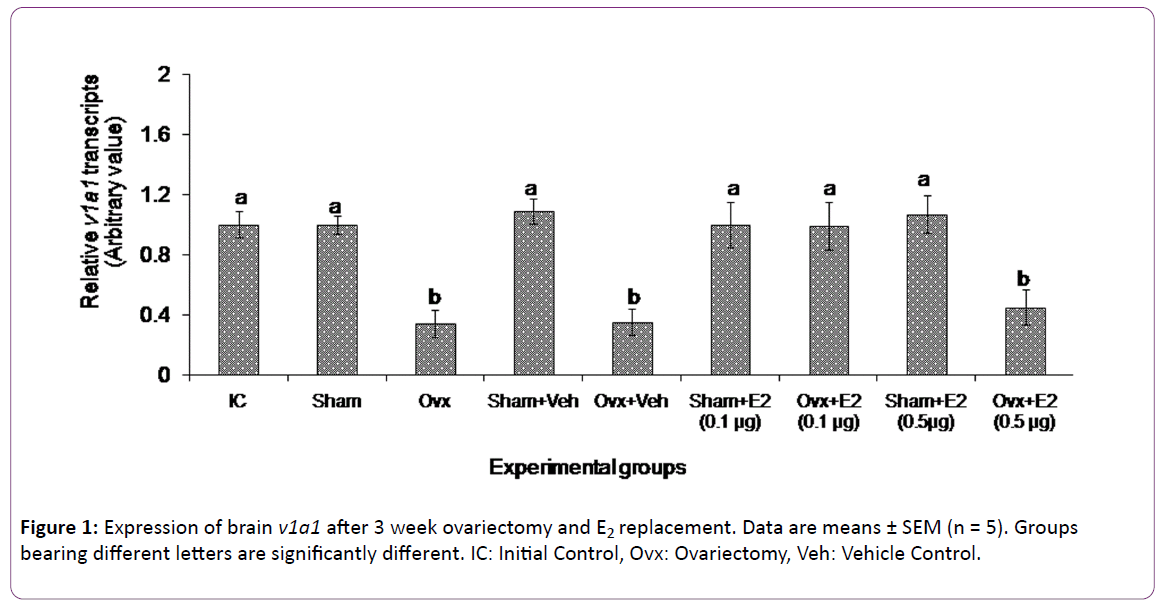
The 3 week ovx and E2 replacement produced overall significant changes in brain v1a1 transcript levels (two way ANOVA, p < 0.001; Fovx = 167.89; FE2 = 34.97; Finteraction = 56.98) (Figure 1). There was no significant change in the v1a1 transcripts in the sham operated group as compared with the initial control group. The 3-week ovx decreased the v1a1 transcripts significantly from the initial control and sham control groups. The low dose (0.1 μg/g BW) of E2 increased and restored the v1a1 transcript levels in the ovx group as compared to the ovx + vehicle group. On the other hand, the high dose (0.5 μg/g BW) of E2 did not alter the v1a1 transcript levels in the ovx group. The E2 administration did not produce any effect on gene expression in the sham control groups.
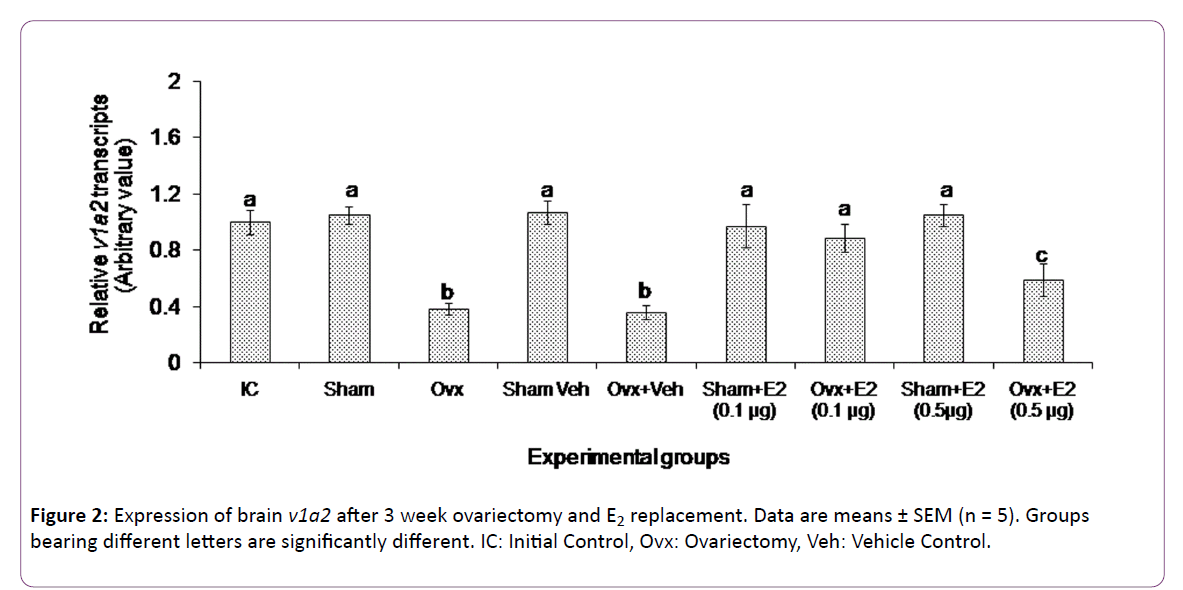
The 3 week ovx and E2 replacement caused overall significant changes in brain v1a2 transcript levels (two way ANOVA, p < 0.001; Fovx = 198.13, FE2 = 24.12, Finteraction = 167.85) (Figure 2). There was no significant change in the v1a2 transcripts in the sham operated group as compared with the initial control group. The 3-week ovx decreased the v1a2 transcripts significantly from the initial control and sham control groups. The low dose of E2 increased and restored the v1a2 transcript levels in the ovx group as compared to the ovx + vehicle group. On the other hand, the high dose of E2 significantly increased the v1a2 transcript levels compared to the ovx groups but did not restore the levels compared to the initial control and sham control groups. The E2 administration did not produce any effect on the gene expression in the sham control groups.
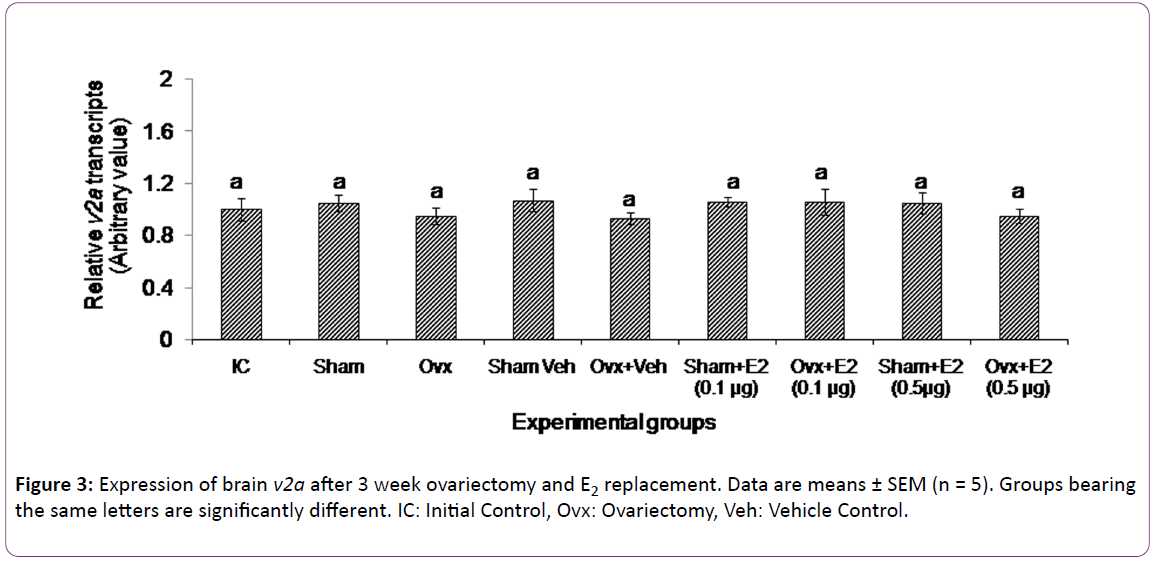
The 3 week ovx and E2 replacement did not alter the expression of v2a receptor (Figure 3).
Discussion
The present study appears to be the first in fishes on the E2 modulation of brain VT receptor genes in a fish species. The data show that both ovx and E2 replacement have elicited differential effects on the VT receptor transcript levels. The V1a type receptor genes (v1a1 and v1a2) responded to both the treatments while neither treatment had any significant effect on the V2 receptor gene expression. Ovariectomy and E2 supplementation are conventional methods used to study E2 feedback effects on gonadotropin secretion [39]. In the catfish, ovx led to a duration-dependent decrease in plasma E2 levels and an increase in plasma gonadotropin levels [36, 37]. The 3 week ovx led to about 3-fold reduction in the transcript levels of v1a1 and v1a2, apparently due to a fall in the E2 level. Thus,ovarian E2 exerts a positive feedback effect on V1a type receptor gene expression. The E2 supplementation study gave further insight into the nature of the feedback. The E2 treatment produced a dose-dependent effect on the expression of both v1a1 and v1a2. The low dose of E2 (0.1 μg) restored the transcript levels to that of the control groups, but the high E2 dose (0.5 μg) did not alter the ovx effect on the v1a1 expression and only slightly elevated the v1a2 expression. Apparently, E2 elicited stimulatory or inhibitory responses on the gene expression depending on the E2 titer. The exogenous E2 administration did not produce any effect in the sham control groups, which may suggest that the steroid treatment may not have any effect on basal transcriptional activity in the presence of endogenous E2. There is a parallel trend in the biphasic effects of E2 on gonadotropin secretion on one hand and VT on the other [25, 26, 36, 37].
There are limited studies on the effect of gonadectomy and steroid treatment on VT receptor activity in vertebrates. Gonadectomy significantly reduced putative receptor concentrations in the amygdala of newt and bullfrog [27, 40]. Estradiol treatment of gonadectomized male and female bullfrogs not only restored receptor concentrations but those levels surpassed the sham levels. Treatment of photorefractory Japanese quail (Coturnix coturnix japonica) with estrogen increased shell gland activity (mass and length of mucosal folds) and levels of both VT and V3 (VT3, oxytocic-like) receptor mRNA, whereas treatment of photosensitive birds with the estrogen antagonist tamoxifen decreased shell gland activity and levels of both VT and VT3 receptor mRNA [28, 41]. In mammals, a positive functional relationship exists between estrogen and uterine OT/OT mRNA/OT receptor [42, 43]. Oxytocin receptor (OTR) is also modulated by E2 in receptor binding studies using 125I-ornithine vasotocin in MCF7 cells, and the upstream palindromic estrogen-response element (ERE) in the rat OR promoter imparts E2 induced OR gene transcription [44]. Tribollet et al. [45] reported that binding of OT to the uterus was estrogen-dependent, and castration and inhibition of aromatase activity reduced, while estradiol and testosterone increased OT binding, particularly in regions of the brain in male and female rats. In the same animals, VP binding in the brain was not affected under those conditions. In sheep, estrogen increases transcription of the OT receptor gene through interaction of estrogen receptor α with a GC rich SP1 enhancer element [43].
Since the V1a type receptor mediates the reproductive function of VT, E2 modulates only the reproductive function of VT and the antiduretic / antidiuretic actions of VT mediated via the V2A receptor are non-responsive to the estrogen status. Since VT has the ring structure of OT and the tail structure of VP, the receptor subtypes through specific signaling mechanisms delineate the reproductive and osmoregulatory functions of the peptide. The estrogen modulation of V1a type receptor in the catfish, the oxytocic VT3 receptor type in birds and the OT receptor type in mammals indicates a phylogenetically conserved basic mechanism of regulation, which is distinct from the regulation of the V2 type receptor gene expression. The distinction is further evident from the fact that these receptor types (V1a, V3/V1b and OT) are coupled to the phospholipase C (PLC)/protein kinase C (PKC) signaling pathway while the V2A type receptor is linked to the cAMP-PKA pathway [13-15]. The receptor subtypes and the specific signalling pathways determine the specific functions of VT, given its multiple tissue targets.
In the catfish brain, the v1a1 and v1a2 transcripts are highly expressed throughout the reproductive cycle and the v2a transcripts are less expressed as shown in the semiquantitative assay [30]. In the breeding phase (preparatory, prespawning and spawning phases), the E2 level fluctuates due to its feedback activity with gonadotropin secretion [36]. The fluctuations in the transcript levels of v1a1 and v1a2 in the brain [30] can be associated with the E2 oscillations, suggesting the existence of a negative feedback control. After spawning, the ovary enters the quiescent phase when the E2 levels are low. The negative feedback mechanism may result in elevated transcript levels especially in the spawning phase. The seasonal pattern of expression of the V1a type receptor gene expression and the response to ovariectomy and E2 treatments suggest that VT engages the v1a1 and v1a2 receptors for the reproductive and behavioral effects. Although we did not see any significant difference in the V1a type and V2a type transcript levels in the qPCR assay in initial control and sham control groups, the v2a expression in the brain may be controlled by other mechanisms, which are to be investigated in future studies. The v2a gene expression is low throughout and not influenced by the E2 oscillations. The present data support this contention. It is not clear at present as to what role neuroestrogens may have on the VT receptor subtype gene expression. It is hypothesized that the neuroestrogens may be related to differential expression of the receptor genes. In the pupfish, v1a1 and v1a2 are most abundant in the midbrain (optic tectum and hypothalamus) and cerebellum in both sexes [11]. In the protogynous bluehead wrasse, the v1a1 transcript is more abundant in telencephalon, hypothalamus, optic tectum, cerebellum and medulla oblongata regions of both male (initial phase and terminal phase) and female fish, while the v1a2 transcript is more abundant in the hypothalamus and cerebellum [12]. VT receptor protein/gene (V1a2/v1a2) has been shown to be distributed widely in the brain of rock hind using immunohistochemical / in situ hybridization technique [46]. These studies indicate that the V1a type receptors are distributed in areas concerned with pituitary regulation, reproductive behavior, olfaction, learning and sensory functions. In the catfish, in situ hybridization study of the three VT receptor subtype genes shows that the v1a paralogs are distributed throughout the brain including the pituitary in high abundance while the v2a type is confined to the anterior dorsal ependymal / subependymal lining of the telencephalon and the pituitary in low levels (our unpublished results). The v2a transcript levels are low throughout the reproductive cycle in the catfish and pupfish [11, 30]. But in medaka and gilthead sea bream, the expression of v2a is high [47, 48]. In the pupfish, acute exposure to hypersalinity (17 ppt or 34 ppt) increased the v1a1 and v1a2 transcript levels but reduced v2a levels in the hypothalamus [11]. In the gilthead sea bream, hyper and hypo osmotic conditions enhanced hypothalamic v2a levels after 7 days of the treatments [48]. The variations in the gene expression in these species may reflect the habitat (freshwater versus marine environment) and habits (breeding pattern, morphe and behavior) and a generalization is difficult to make due to lack of comparative studies. Alternatively, different fishes may use different receptor gene paralogs to mediate VT actions.
Although we did not analyze the VT receptor subtype gene expression separately, the transcript abundance is generally low in the pituitary compared to the brain regions [30]. The transcript abundance of v1a1 and v1a2 is the highest in the rostral pars distalis of the pituitary while v2a has relatively low levels (unpublished in situ hybridization results). In the pupfish, the expression of v1a1 and v2 is high in the pituitary,but that of v1a2 is low [11]. In the gilthead sea bream, v2 transcripts are highly expressed in the pituitary with low v1a2 levels [48]. The pituitary is the major target of VT fibers from which the hormone is released into circulation [31]. Additionally, the VT fibers directly innervate the adenohypophysial cells suggesting VT modulation of the hormone secretion. Fryer and Leung, reported corticotropin (ACTH) releasing activity of VT and IT [49]. Pierson et al. [50] reported that the VT stimulation of ACTH was through the mediation of the V1 type receptor. VT has been shown to stimulate gonadotropin release in Poecilia latipinna[51]. In Cichlasoma dimerus, a species with alternative phenotypes, VT stimulated LH (dose-related effects) and FSH secretion in pituitary incubates, and androgen release from testicular fragments [52]. In the catfish, the differential response of the VT receptors to estrogen levels suggests VT control of gonadotropic activity via the V1a type receptor. In conclusion, estrogens may modulate reproductive and behavioural responses partly through VT actions via the V1a type receptors.
Acknowledgement
This work was supported by a research grant of Department of Science and Technology, New Delhi (Grant No. SA/SO/ AS-43/2009) awarded to KPJ and RC. We are grateful to Prof. M. K. Thakur, Coordinator of DBT-BHU ISLS for the qPCR facility extended to us.
References
- Amer S, Brown JA (1995) Glomerular actions of arginine vasotocin in the in situ perfused trout kidney, American journal of physiology. Regulatory, integrative and comparative physiology 269: 775-780.
- Acher R,Chauvet MT, Chauvet MG, Rouille Y (1997) Molecular evolution of neurohypophyseal hormones in relation to osmoregulation: the two fish option. Fish Physiology and Biochemistry 1: 325-332.
- Bradshaw SD, Bradshaw FJ (2002) Mini-review - Arginine vasotocin: site and mode of action in the reptilian kidney. General and Comparative Endocrinology 126: 7-13.
- Wells A, Anderson WG, Hazon N, (2002) Development of an in situ perfused kidney preparation for elasmobranch fish: action of arginine vasotocin.American Journal of Physiology 282: 1636-1642.
- Balment RJ, Lu EW, Weybourne W, Warne JM, (2006) Arginine vasotocin: a key hormone in fish physiology and behaviour: A review with insights from mammalian models. General and Comparative Endocrinology 147: 9-16.
- Joy KP, Chaube R (2015)Vasotocin- A new player in the control of oocyte maturation and ovulation in fish. General and Comparative Endocrinology221: 54-63.
- Lolait SJ, O’CarrolAM,McBride,OW,Konig,M, Morel, et al. (1992) Cloning and characterization of a vasopressin V2 receptor and possible link tonephrogenic diabetes insipidus. Nature357: 336-339.
- Morel A, O’Carroll AM, Brownstein MJ, Lolait SJ (1992) Molecular cloning and expression of rat V1a arginine vasopressin receptor. Nature 356: 523-526.
- Sugimoto T, Saito M, Mochizuki S, Watanabe Y, Hashimoto S, et al. (1994). Molecular cloning and function expression of a cDNA encoding the human V1b receptor vasopressin receptor.Journal of Biological Chemistry269: 27088-27092.
- Hasunuma I, Sakai T, Nakada T, Toyoda F, Namiki H, et al. (2007) Molecular cloning of three types of arginine vasotocin receptor in the newt, Cynopspyrrhogaster. General and Comparative Endocrinology151: 252-258.
- Lema SC, (2010) Identification of multiple vasotocin receptor cDNAs in teleost fish: sequences, phylogenetic analysis, sites of expression, and regulation in the hypothalamus and gill in response to hyperosmotic challenge. Mol Cell Endocrinol 321: 215-230.
- Lema SC, SlaneMA, SalvesenKE, GodwinJ(2012) Variation in gene transcript profiles of two V1a-type arginine vasotocin receptors among sexual phases of blueheadwrasse (Thalassomabifasciatum). General and Comparative Endocrinology179: 451-464.
- Gimpl G, FahrenholzF (2001) The oxytocin receptor system: structure, function, and regulation. Physiological Reviews 81: 629-683.
- OcampoDaza D, Lewicka M, LarhammarD (2012) The oxytocin/ vasopressin receptor family has at least five members in the gnathostome lineage, including two distinct V2 subtypes. General and Comparative Endocrinology.175: 135-143.
- Yamaguchi Y, Kaiya H, Konno N, Iwata E, Miyazato M, et al. (2012) The fifth neurohypophysial hormone receptor is structurally related to the V2-type receptor but functionally similar to V1-type receptors. General and Comparative Endocrinology178: 519-528.
- Lagman D, OcampoDaza D, WidmarkJ,AbaloXM,Sundstrom G, et al. (2013). The vertebrate ancestral repertoire of visual opsins, transducin alpha subunits and oxytocin/ vasopressin receptors was established by duplication of their shared genomic region in the two rounds of early vertebrate genome duplications. BMC Evolutionary Biology 13: 238.
- Voorhuis TAM, Kiss JK, de Kloet ER, de WiedD (1988).Testosterone-sensitive vasotocin-immunoreactive cells and fibers in the canary brain. Brain Research 442: 139-146.
- Adan RAH,Burbach JPH (1992) Regulation of vasopressin and oxytocin gene expression by estrogen and thyroid hormones.Progress in Brain Research 932: 127-136.
- Miller MA, De Vries GJ, Hussien A, Shamma Al, Dorsa MD, (1992) Decline of vasopressin immunoreactivity and mRNA levels in the bed nucleus of striaterminalis following castration.Journal of Neuroendocrinology12: 2281-1887.
- Moore FL (1992) Evolutionary precedents for behavioural actions of oxytocin and vasopressin. Annals of the New York Academy of Sciences 652: 156-165.
- Boyd SK (1994)Arginine vasotocin facilitation of advertisement calling and call phonotaxis in bullfrogs. Hormones and Behavior 25: 232-240.
- Ota Y, Ando H, Ban M, Ueda H, Urano A (1996) Sexually different expression of neurohypohysial hormone genes in the pre-optic nucleus of the pre-spawning chum salmon. Zoological Science 13: 593-601.
- Ota Y, Ando H, Ueda H, Urano A (1999) Differences in seasonal expression of neurohypophysial hormone genes in the preoptic nucleus of immature female Masu salmon. General and Comparative Endocrinology116: 40-48.
- Urano A, Kubokawa K, HiraokaS (1994)Expression of the vasotocin and isotocin gene family in fish. In: Sherwood, NM and Hew CL (Eds.) ‘‘Fish Physiology’’, Academic Press, New York, pp: 101-132.
- Singh V, Joy KP (2009a) Effects of hCG and ovarian steroid hormones on vasotocin levels in the female catfish Heteropneustesfossilis.General and Comparative Endocrinology162: 172-178.
- Chaube R, Singh RK, Joy KP (2012) Estrogen regulation of brain vasotocin secretion in the catfish Heteropneustesfossilis: an interaction with catecholaminergic system. General and Comparative Endocrinology 175: 206-213.
- Boyd SK, Moore FL (1991)Gonadectomy reduces the concentrations of putative receptors for arginine vasotocin in the brain of an amphibian.Brain Research 541: 193-197.
- Srivastava R, Cornett LE,Chaturvedi CM (2007)Effect of photoperiod and estrogen on expression of arginine vasotocin and its oxytocic-like receptor in the shell gland of the Japanese quail. Comparative Biochemistry and Physiology Part A: Molecular &Integrative Physiology 148: 451-457.
- Grozhik AV, Horoszko CP, Horton BM, Hu Y, Voisin DA, et al. (2014) Hormonal regulation of vasotocin receptor mRNA in a seasonally breeding songbird. Hormones and Behavior65: 254-263.
- RawatA,Chaube R,Joy KP, (2015) Molecular cloning, sequencing and phylogeny ofvasotocinreceptor genes in the air-breathing catfish Heteropneustesfossilis with sex dimorphic and seasonal variations in tissue expression.Fish Physiology and Biochemistry 41: 509-532.
- Singh V, Joy KP (2008) Immunocytochemical localization, HPLC characterization and seasonal dynamics of vasotocin in the brain, blood plasma and gonads of the catfish Heteropneustesfossilis.General and Comparative Endocrinology159: 214- 225.
- Singh V, JoyKP (2009b)Relative in vitro seasonal effects of vasotocin and isotocin on ovarian steroid hormone levels in the catfish Heteropneustesfossilis. General and Comparative Endocrinology162: 257-264.
- Singh V, Joy KP (2010)An involvement of vasotocin in oocyte hydration in the catfish Heteropneustesfossilis: A comparison with effects of isotocin and hCG. General and Comparative Endocrinology 166: 504-512.
- Singh V, JoyKP(2011)Vasotocininduces finaloocyte maturation and ovulation through the production of a maturation-inducing steroidin the catfish Heteropneustesfossilis. General and Comparative Endocrinology174: 15-21.
- Joy KP,Singh V (2013) Functional interactions between vasotocin and prostaglandins during final oocyte maturation and ovulation in the catfish Heteropneustesfossilis.General and Comparative Endocrinology 186: 126-135.
- Senthilkumaran B, JoyKP (1994) Effects of ovariectomy and oestradiol replacement on hypothalamic serotonergic and monoamine oxidase activity in the catfish, Heteropneustesfossilis: a study correlating plasma oestradiol and gonadotrophin levels.Journal of Endocrinology142: 193-203.
- Senthilkumaran B, Joy KP (1995) Changes in hypothalamic catecholamines, dopamine-ß-hydroxylase and phenylethanolamine-N-methyltrasferase in the catfishHeteropneustesfossilisin relation to season, raised photoperiod and temperature, ovariectomy and estradiol-17-ß replacement.General and Comparative Endocrinology97: 121-134.
- Livak KJ, SchmittgenTD (2001)Analysis of relative gene expression data using real-time quantitative PCR and the 2-DDCT method. Methods 25: 402-408.
- Goos HJT, Senthilkumaran B, Joy KP (1999)Neuroendocrine integrative mechanisms in the control of gonadotropin secretion in teleosts. In: Joy KPKrishna, A Haldar C (Eds)Comparative Endocrinlogy and Reproduction, New Delhi, Berlin: Narosa, Springer-Verlag, pp: 113-136.
- Boyd SK (1997) Brain vasotocin pathways and the control of sexual behaviors in the bullfrog.Brain Research Bulletin 44: 345-350.
- Srivastava R, Cornett LE, ChaturvediCM (2008)Effect of estrogen and its antagonist on the expression of arginine vasotocin (AVT) and its oxytocic-like receptor VT3 in the shell gland of Japanese quail, Coturnixcoturnix japonica. Comparative Biochemistry and Physiology Part A: Molecular &Integrative Physiology 151: 551-559.
- Larcher A, Neculcea J, Breton C, Arsian A, RozenF, et al. (1995) Oxytocin receptor gene expression in the rat uterus during pregnancy and the estrous cycle and in response to gonadal steroid treatment. Endocrinology 136: 5350-5356.
- Fleming GW, Spencer TE, Safe SH, Bazer FW (2006) Estrogen regulates transcription of the ovine oxytocin receptor gene through GC rich SP1 promoter elements. Endocrinology 14: 899-911.
- Bale TL, Dorsa DM (1997) Cloning, novel promoter sequence, and estrogen regulation of a rat oxytocin receptor gene. Endocrinology 138: 1151-1158.
- Tribollet E,Audigier S, Dubois-Dauphin M,Dreifuss JJ (1990) Gonadal steroids regulate oxytocin receptors but not vasopressin receptors in the brain of male and female rats, An autoradiographical study. Brain Research511: 129-140.
- Kline JR, O’Connell AL, Hofmann AH, Holt JG, KhanAI(2011) The distribution of an AVT V1a receptor in the brain of a sex changing fish Epinephelusadscensionis.Journal of Chemical Neuroanatomy 42: 72-88.
- Konno N,KurosawaM,KaiyaH,MiyazatoM,MatsudaK et al.(2010) Molecular cloning and characterization of a V2-type receptor in two ray-finned fish, gray bichir, Polypterussenegalus and medakaOryziaslatipes.Peptides3: 1273-1279.
- Martos-Sitcha JA, FuentesJ, Mancera JM, Martinez-Rodriguez G(2014) Variations in the expression of vasotocin and isotocin receptor genes in the gilthead sea bream Sparusaurata during different osmotic challenges. General and Comparative Endocrinology197: 5-17.
- Fryer J, LeungE (1982)Neurohypophyseal hormonal control of cortisol secretion in the teleost,Carassiusauratus. General and Comparative Endocrinology48: 425-431.
- Pierson PM, Guibbolini ME, Lahlou B (1996) A V1-type receptor for mediating the neurohypophysial hormone induced ACTH release in trout pituitary. Journal of Endocrinology 149: 109-115.
- Groves DJ, Battenn TFC (1986)Direct control of the gonadotroph in a teleost Poecilialatipinna. II. Neurohormones and neurotransmitters. General and Comparative Endocrinology 62: 315-326.
- Ramallo M, Grober M, Pandolfi M (2011) Effects of arginine vasotocin on the hypothalamic-pituitary-gonads axis: A behavioural approach. Indian Journal of Science and Technology 4: 15-16.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences