In situ Localization of Vasotocin and Isotocin Precursor mRNA in Brain and Ovary of the Catfish Heteropneustes Fossilis and Estrogen Regulation of the Gene Expression
Banerjee P, Chaube R, Joy KP
Banerjee P1, Chaube R1 and Joy KP2*
1Department of Zoology, Centre of Advanced Study, Banaras Hindu University, Varanasi-221005, India
2Department of Biotechnology, Cochin University of Science and Technology, Kochi-682022, India
- *Corresponding Author:
- Joy KP
INSA Senior Scientist, Department of Biotechnology
Cochin University of Science and Technology, Kochi-682022, India
Tel: 918281274891
E-mail: kpjoybhu@gmail.com
Received date: November 23, 2015; Accepted date: January 04, 2016; Published date: January 07, 2016
Citation: Banerjee P, Chaube R, Joy KP. In situ Localization of Vasotocin and Isotocin Precursor mRNA in Brain and Ovary of the Catfish Heteropneustes Fossilis and Estrogen Regulation of the Gene Expression. J Transl Neurosci. 2016, 1:1
Copyright: © 2016 Banerjee P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
In the catfish, vasotocin and isotocin precursor (pro-VT and pro-IT) transcripts were localized in brain and ovary by RNA in situ hybridization and estrogen control of gene expression studied by qRT-PCR. Both pro-VT and pro-IT transcripts were detected in nucleus preopticus (NPO) of the brain. Within the NPO, the VT and IT neurons displayed distinct spatial distribution and neuronal organization. Apart from the NPO, pro-VT transcripts were also detected in the anterior nucleus lateralis tuberis. In the ovary, the follicular envelope (theca and granulosa layers) of ovarian follicles showed strong positive transcript signals, which was reported for the first time in teleosts. The regulation of expression of pro-VT and pro-IT genes by estrogen was investigated in vitro using brain preoptic area and ovarian slices in presence of exemestane, a known endogenous estrogen (E2) synthesis blocker, alone or in co-incubation with E2. Exemestane elicited differential effects on the nonapeptide expression in the brain and ovary. Exemestane mildly downregulated pro-IT expression but did not influence the pro-VT expression in the brain. In the ovary, exemstane inhibited both pro-VT and pro-IT expression about 4 folds higher than the control groups. The E2 supplementation increased the expression of both pro-VT and pro-IT in the brain differentially with the high E2 dose eliciting about 3-fold stimulation of the pro-VT expression. In the ovary, only the high dose of E2 supplementation restored and increased the pro-VT and pro- IT expression compared to the exemstane group. The steroid-induced expression was still significantly lower than the control level. The presence of pro-VT and pro-IT transcripts in the follicular envelope points to de novo synthesis of the neuropeptides in the ovary, which is influenced by the locally synthesized E2. The pro-VT and pro-IT neurons seem to be responsive to the E2 feedbacks rather than to the locally produced E2.
Keywords
Catfish; Estrogen; Exemstane; Gene expression; in situ hybridization; Nonapeptides
Introduction
The nonapeptides are a family of structurally-related peptide hormones with conserved structural features of two half-cysteine residues at 1 and 6, an amidated glycine at the 9th position and varying amino acid residues at 3, 4 and 8 positions [1]. The nonapeptides are classified into two families: basic and neutral families based on the amino acid at the 8th position. In teleosts, the basic and neutral nonapeptides are vasotocin (VT) and isotocin (IT), respectively, and are synthesized in distinct neurons in the nucleus preopticus (NPO) and axonally transported to the neurohypophysis from where they are released into the circulation [2]. The circulating nonapeptides perform major functions like osmoregulation, reproduction, metabolism, behavior, cardiovascular function etc., [3] mediated through specific receptors [4]. In addition to the brain, nonapeptides are also synthesized in peripheral organs like ovary, testis, uterus, adrenal, thymus, pancreas, etc., of higher vertebrates [5-11]. Such studies are meager in lower vertebrates. Using immunohistochemical and HPLC methods, VT was characterized in the follicular envelope of ovarian follicles in the catfish
Heteropneustes fossilis [12]. The ovarian VT shows seasonal and periovulatory changes and is involved in steroidogenesis and prostaglandin secretion, and follicular hydration, maturation and ovulation [12-17]. In the testis, VT secretion is relatively low and does not show any seasonal significant variation [12]. Ramallo et al. [18-20] demonstrated VT immunoreactivity in the interstitial tissue of the testis of the cichlid Cichlasoma dimerus. In rainbow trout [21] reported that VT stimulates In vitro testosterone production by the immature testis. However, information on the role of IT is scarce and it has a lesser role in reproductive functions [13,14,22]. The non-availability IT-specific antibody is a constraint for localization and expression studies. Bobe et al. [23] reported a high expression of both pro-VT and pro-IT genes in rainbow trout during oocyte maturation. In the catfish, cloning and characterization of pro- VT and pro-IT genes showed that both genes are expressed exclusively in the brain and gonads [24]. The development of molecular probes in the catfish has enabled the study of nonapeptides at the gene level.
Gonadal steroid hormones are involved in the regulation of nonapeptide secretion in vertebrates [25-29]. Brain pro-VT and pro-IT mRNA levels were correlated with sex steroid hormone levels in salmons [2]. In the catfish, ovariectomy decreased brain and plasma VT levels and estradiol-17β (E2) replacement in 3-week ovariectomized fish elicited a dose-dependent increase in the VT levels [30-33]. E2 produced season- and dose-dependent effects on VT secretion in the catfish ovary In vitro [30]. While, in the preparatory phase, E2 produced a dose-dependent modulation: low dose stimulated, and high dose inhibited VT levels, in the prespawning phase, E2 inhibited VT secretion in a dose-dependent manner. The question whether gonadal steroids modulate pro-VT and pro-IT gene expression is not investigated so far. Further, the teleost brain is an important source of neurosterogens [34]. The catfish brain expresses both ovarian and brain type P450 aromatase genes and synthesizes E2 [33,35]. The role of neuroestrogens in the modulation of nonapeptide gene expression and peptide secretion is not yet investigated although estrogen receptors are occur on the nonapeptide neurons [36,37]. Ovariectomy and E2 replacement can, at best, show the involvement of peripheral estrogens on nonapeptide secretion.
Keeping the above in mind, in the present study we demonstrated the cellular localization of pro-VT and pro-IT transcripts in the brain and ovary of the catfish to confirm de novo peptide synthesis. Secondly, we used an In vitro model system to demonstrate the role of locally produced E2 on the expression of pro-VT and pro-IT. For this, the preoptic area (POA) and ovary slices were incubated with exemstane, an irreversible steroidal inhibitor of P450 aromatase [38], to block endogenous E2 synthesis and the gene expression was measured in the presence of the inhibitor and E2 supplementation.
Material and Methods
Animal collection and acclimation
Heteropneustes fossilis (40–50 g) were collected from local fish markets in Varanasi in January (resting phase) and mid-May (pre-spawning phase). They were maintained in the laboratory for 48 h under natural photoperiod: January (11.0L:13.0D), May (13.0L:11.0D) and temperature (25 ± 2°C) to overcome stress due to transportation and fed daily with goat liver ad libitum. All experiments were performed in accordance with the guidelines of the Animal Ethics Committee, Banaras Hindu University, Varanasi.
Chemicals and reagents
Guanidine thiocyanate-phenol solution (Qiagen), Revert-Aid H Minus First Strand cDNA Synthesis Kit (Fermentas), 2X PCR Master Mix (Fermentas), Nucleo-pore PCR clean- up gel extraction kit (Genetix), InsTAclone PCR Cloning Kit (Thermo), Maxiscript In vitro transcription kit (Ambion), dextran sulfate (Amresco), Denhardt’s reagent (Invitrogen), veriquest SYBR green qPCR master mix (Affymetrix) and DNase I (Ambion) were purchased through local suppliers. Dig-11 UTP, yeast t-RNA, blocking reagent, antidigoxigenin AP Fab fragments and NBT/ BCIP stock solution were purchased from Roche. Agarose, tris base, glacial acetic acid, EDTA–Na2, proteinase K and other chemicals were of molecular grade, purchased from E-Merck, Mumbai. LB broth, LB agar, ampicillin, X-Gal and IPTG were purchased from Hi Media, Mumbai. Diethyl pyrocarbonate (DEPC), 3-aminopropyl triethoxysilane, formamide (molecular biology grade) and estradiol-17β (E2) were purchased from Sigma-Aldrich, New Delhi. Exemestane (FCE-24304) with the trade name of aromasin (Pfizer, Italy) was purchased from a local medical store. The primers used were synthesized by Integrated DNA Technology (IDT), Faridabad, India.
Cloning and generation of probes
Heteropneustes fossilis VT (acc. no. JX035928.1) and IT (acc. no. JX669009.1) precursor genes were cloned and used for the generation of riboprobes. The pro-VT (505 bp) and pro-IT (569 bp) were amplified by PCR using the primers spanning the 5’ and 3’ UTR regions (Table 1) and the brain cDNA as the template. The pro-VT and pro- IT share an identity of only 53.47% so that the probes are expected not to cross-react. The PCR products were cloned into the TA cloning vector pTZ57R/T of InsTAclone™ PCR Cloning Kit (Thermo), using the manufacturer’s protocol and competent cells of DH5α strain of E coli. Plasmids were extracted from the recombinant (white) colonies and screened for the orientation of ligation of the PCR product by using a PCR strategy. Briefly, the protocol included using a VT and IT UTR FP/ UTR RP and T7 promoter primer and the extracted plasmid as template in a PCR. Plasmids giving a positive amplification with VT/IT UTR FP and T7 primer were used for the generation of antisense probes. Plasmids giving a positive amplification with the VT/IT UTR RP and T7 primer were used for the generation of sense probes. These PCR products were purified using the Nucleo-pore PCR clean- up gel extraction kit (Genetix) and 250 ng of the products were used as the template for In vitro transcription using MAXIscript® T7 In vitro transcription kit (Ambion). The In vitro transcription reaction was carried out in a 20 μL reaction volume using T7 polymerase and 1 μL of 10 mM stock each of ATP, CTP, GTP and UTP. In this reaction, 1 μL of digoxigenin-11-UTP (Roche) (3.5 mM stock) was used as the labeled UTP. The reaction was carried out at 37°C for 1 h. 1 μL of the resulting reaction was checked by agarose gel electrophoresis to confirm the formation of the riboprobes and stored at -80°C for in situ hybridization.
| Primers | Sequences |
|---|---|
| VT FP | TGTTACATCCAGAACTGCCCCAGA |
| VT RP | CAGCCCAGTCCTTCTCCACAGCA |
| VT H FP | GTTACATCCAGAACTGCCCCAGA |
| IT FP | TCAATCTTCTGCATGCTGTGTCT |
| IT RP | CACACGCCATGCACTGTCTATTG |
| IT H FP | ACATCTCCAACTGTCCCATC |
| β actin FP | TGGCCGTGACCTGACTGAC |
| β actin RP | CCTGCTCAAAGTCAAGAGCGAC |
| T7 primer | TAATACGACTCACTATAGGG |
| VT UTR FP | GTCCAGTGAGAGACAGACCTCCGG |
| VT UTR RP | TAGAATGGACCGCGTGCTCTGC |
| IT UTR FP | CATCAGCTACTGAAGCTACTGATTCGT |
| IT UTR RP | AGGACATCAGAAGGTTCGGCTG |
Table 1: Details of the primers used for generating the riboprobes.
in situ hybridization
in situ hybridization was performed in both whole brain and tissue sections. All solutions and reagents were prepared in diethyl pyrocarbonate (DEPC)-treated water to maintain RNase- free conditions at all steps prior to hybridization. The procedure for whole brain in situ hybridization (WISH) and in situ hybridization on tissue sections was almost the same, only differing in tissue pretreatment, composition of hybridization buffers and posthybridization washing solution (supplementary Table 1). The brains dissected out from the resting phase fish were washed in ice - cold phosphate buffered saline (PBS) and allowed to harden in 4% paraformaldehyde (PFA) for 3 h. The hardened tissues were trimmed to remove the cerebellum and medulla oblongata and were put into fresh 4% PFA to fix overnight at 4°C. For the WISH on the fixed tissues, the protocol of [39] was followed with some modifications. In short, the fixed tissues were first washed in PBS/ 0.01% Tween- 20 (PBS-T), dehydrated through a series of methanol diluted in the PBS-T at room temperature (RT). The last step (100% methanol treatment) was repeated once and the tissues were stored in 100% methanol at -20°C overnight. The dehydrated tissues were rehydrated, washed in the PBS-T, digested with proteinase K (10 μg/mL) for 15 min, refixed in 4% PFA, washed in the PBS-T, pre-hybridized at 70°C in hybridization buffer without probe, followed by hybridization with probes (sense/antisense) diluted 1:500 in the hybridization buffer overnight at 70°C. Overnight hybridization was followed by stringency washes by incubating the tissues in successively lower salt solutions at the hybridization temperature. Next, the tissues were washed in 1 x maleic acid buffer-0.1% tween 20 (MAB-T) at RT, blocking in blocking solution for 2 h, then incubated in 1:2000 diluted anti-digoxigenin AP antibody (Roche) at 4°C. The antibody incubation was followed by washing in MAB-T and alkaline phosphatase buffer. Detection was done by applying a 1% solution of the NBT/BCIP stock solution (Roche) prepared in the alkaline phosphatase buffer and allowing the reaction to proceed in dark for 40 min (the reaction development time was standardized for optimal intensity with the least background).
For in situ hybridization on tissue sections, the fixed tissues were dehydrated in graded series of ethanol. 100% ethanol was replaced by xylene, followed by a 50:50 mixture of xylene and paraffin wax before embedding. Eight μm thick transverse and sagittal sections of the brain were cut on a Leica semi-automatic microtome and spread on silane-coated slides to affix the sections. The ovary was sectioned in the transverse plane. The slides were dewaxed in xylene and processed similarly as the whole brain for hybridization, washing, antibody incubation (carried out at RT instead of 4°C) and detection.
In vitro effects of exemestane and E2 supplementation
Preparation of test compounds and incubation medium- A stock solution of E2 was made in propylene glycol after first dissolving the required amount in 50 μL of ethanol. A stock solution of exemestane was prepared in a 1:1 solution of PBS:DMSO, after dissolving the required amount in 100 μL of ethanol. Just before the incubation, the stock solutions were diluted with the incubation medium to make working concentrations. Leibovitz’s L-15 medium (AT011A-1L, Hi Media Laboratories Pvt. Ltd., Mumbai, India), supplemented with 10% fetal bovine serum and 100 μg/mL streptomycin was prepared for the In vitro study. The medium was always prepared fresh and filtered in a sterilized syringe-driven filter having a nylon hydrophilic membrane of pore size 0.45 μm and 30 mm diameter before use.
In vitro incubations of brain and ovary- The organs were removed under aseptic conditions quickly from adult female fish in the pre-spawning phase after decapitation. The dissected brain and ovary were washed in ice-cold fish saline. For the preparation of brain slice, an anterior cut was made at the level of the anterior commissure to remove the telencephalic area and a posterior cut was made behind the optic chiasm with the help of a clean sharp razor blade. This slice contained the POA with the nucleus preopticus (NPO) and was used for the experiment. A slice from the middle part of the ovary was used for the experiment. Five treatment groups were made each for brain and ovary incubations: Group 1 (vehicle control), the tissue slices were incubated with the vehicle for 8 h. Group 2 (EM) was incubated with exemestane (200 nM) alone for 8 h. Group 3 (EM + vehicle) was incubated with exemestane (200 nM) for 3h and then coincubated with exemestane and propylene glycol. Group 4 (EM + low E2) was incubated with exemestane (200 nM) for 3 h and then co-incubated with exemestane and 1 nM E2 for 5 h. Group 4 (EM+ high E2) was incubated with exemestane (200 nM) for 3 h and then co-incubated with exemestane and 10 nM E2 for 5 h. The incubations were carried out in 5 mL of culture medium at 25°C in a CO2 incubator with 5% CO2. Five fish were used in each group. The exemestane dose was chosen, as reported in [40] for fish ovarian tissue. The dose is about 100 times more than the IC50 (concentration giving 50% inhibition) values for the inhibition of human Cyp191a1 [41]. After the incubation period, the tissues were collected in RNA later and stored at -20°C until further processing.
qPCR for nonapeptide precursor gene expression
A two-step qPCR was conducted to study the expression of pro- VT and pro-IT genes. Total RNA was isolated by the single-step method of RNA isolation. To remove genomic DNA contamination from the preparation, DNAase (Ambion) treatment (2 units/10 μg RNA) was given and subsequently DNAase was heat inactivated at 75°C in presence of EDTA. RNA purity was checked by A260/A280 ratio. Samples having a ratio above 1.8 were only considered for reverse transcription. Two μg of the total RNA was reverse transcribed using random hexamer primers and Revert Aid M-MuLV reverse transcriptase in a 20 μL reaction volume (first strand cDNA synthesis kit, Fermantas) using the manufacturer’s protocol. The resulting cDNA was diluted 10 times and 1μL was used in a PCR reaction of 20 μL containing veriquest SYBR green 2X master mix, VT/IT FP and IT FP/ RP, using the manufacturer’s protocol in an Applied Biosystem 7500 machine with a thermal condition of 50º C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. The specificity of the PCR product was checked by dissociation curve analysis of the amplicon and was also checked by agarose gel electrophoresis. The relative gene expression in the different experimental groups was calculated using the comparative CT method with catfish β-actin (accession number FJ409641.2) used as the endogenous control. The vehicle control cDNA was used as the calibrator sample. Each reaction was set up in duplicate and the average CT value was taken for the calculation. Graphs were plotted with the mean RQ (relative quantity) values (2-ΔΔCT) as calculated from five fish each [42].
Statistical analysis
Data were expressed as mean ± SEM and were analyzed for statistical significance by one way ANOVA (p < 0.001), followed by Newman-Keuls’ test (p < 0.05).
Results
Localization of pro-VT and pro-IT transcripts in brain
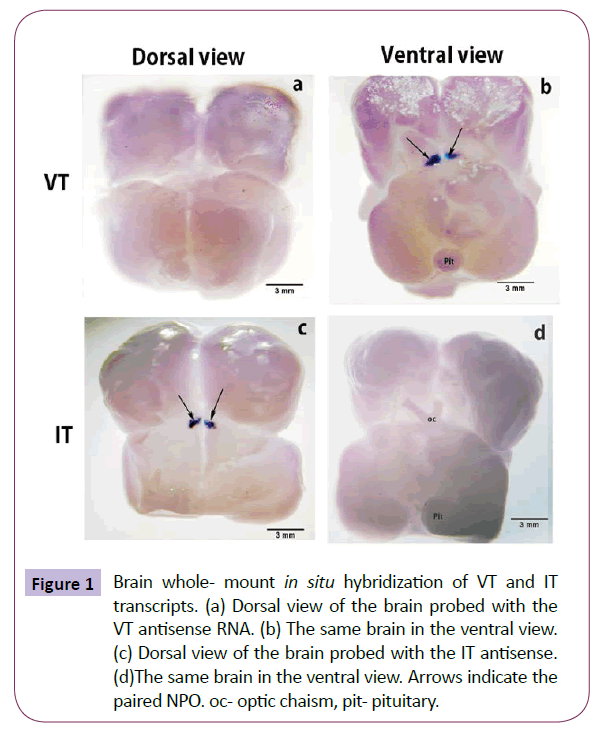
The whole brain preparation demonstrated the gross distribution of the precursor genes in the NPO (Figure 1). Within the NPO, the pro-VT and pro-IT transcripts exhibited a spatial distribution. The pro-VT neurons were present in the ventral part of the NPO, and not seen from the dorsal side (Figures 1a and 1b). The pro- IT neurons were present on the dorsal part of the NPO, and not seen from the ventral part (Figures 1c and 1d).
Figure 1: Brain whole- mount In situ hybridization of VT and IT transcripts. (a) Dorsal view of the brain probed with the VT antisense RNA. (b) The same brain in the ventral view. (c) Dorsal view of the brain probed with the IT antisense. (d)The same brain in the ventral view. Arrows indicate the paired NPO. oc- optic chaism, pit- pituitary.
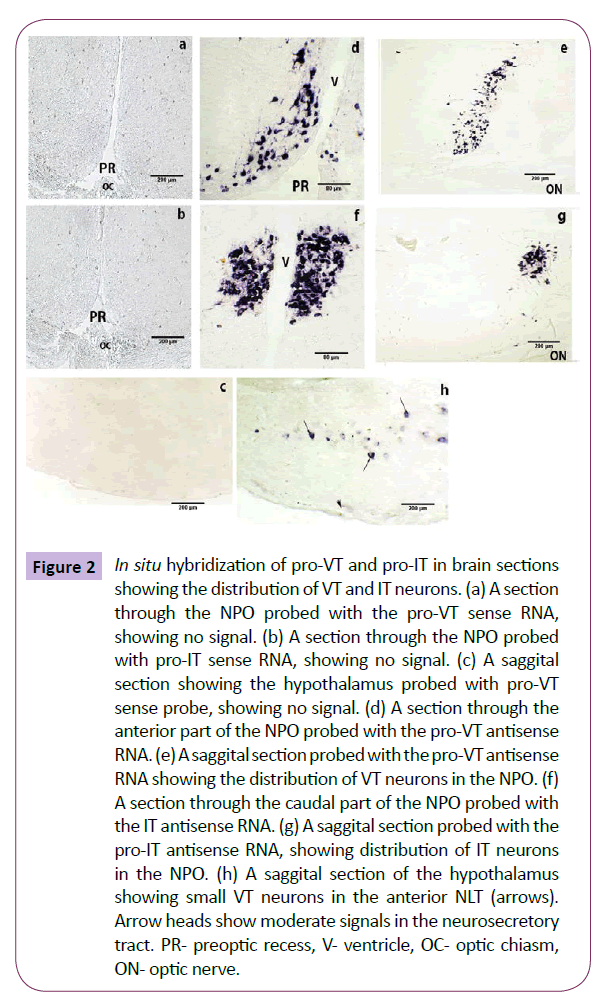
The brain sections hybridized with the sense probe for pro-VT and pro-IT did not give any positive signal (Figures 2a-2c). The regional distribution of pro-VT and pro-IT transcripts was clearly evident on probing the sections with the antisense pro-VT and pro-IT RNA probes. In the catfish, the NPO can be divided into two divisions: [ventral pars parvocellularis (ppc) and dorsal pars magnocellularis (pmc)]. The pro-VT neurons are distributed in both the subdivisions; smaller neurons in the NPO-ppc and larger neurons in the NPO-pmc (Figures 2d and 2e). The examination of the NPO in serial sections from the anterior to the posterior end shows that the VT neurons extend along the antero-ventral region of the NPO-ppc laterally to a more dorso-medial NPOpmc caudally. The pro-IT neurons occupy the dorsal aspect of the NPO, throughout the rostro-caudal axis (Figures 2f and 2g). The magnocellular subdivision has more IT neurons than the parvocellular subdivision. In addition to the NPO, pro-VT signal was also noticed in the ventral hypothalamus in a few neurons of the anterior nucleus lateralis tuberis (NLT) (Figure 2h).
Figure 2: In situ hybridization of pro-VT and pro-IT in brain sections showing the distribution of VT and IT neurons. (a) A section through the NPO probed with the pro-VT sense RNA, showing no signal. (b) A section through the NPO probed with pro-IT sense RNA, showing no signal. (c) A saggital section showing the hypothalamus probed with pro-VT sense probe, showing no signal. (d) A section through the anterior part of the NPO probed with the pro-VT antisense RNA. (e) A saggital section probed with the pro-VT antisense RNA showing the distribution of VT neurons in the NPO. (f) A section through the caudal part of the NPO probed with the IT antisense RNA. (g) A saggital section probed with the pro-IT antisense RNA, showing distribution of IT neurons in the NPO. (h) A saggital section of the hypothalamus showing small VT neurons in the anterior NLT (arrows). Arrow heads show moderate signals in the neurosecretory tract. PR- preoptic recess, V- ventricle, OC- optic chiasm, ON- optic nerve.
Neuron morphology and contacts
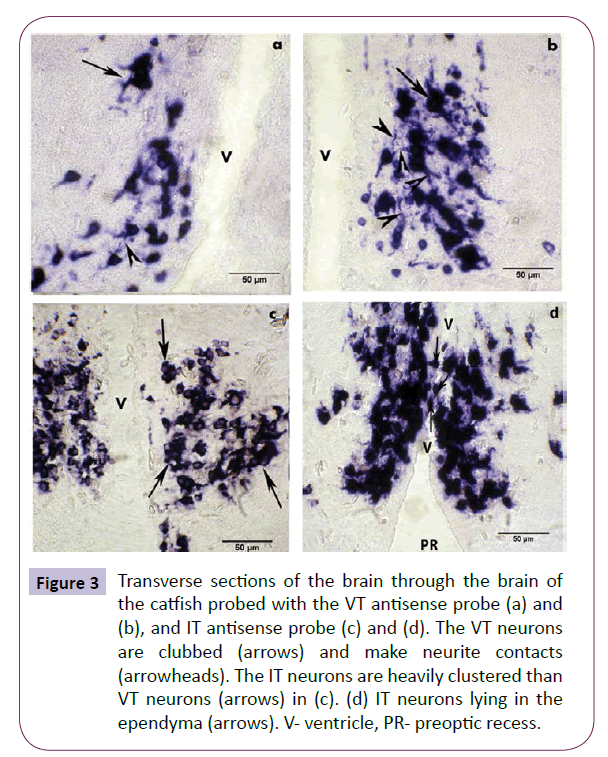
In the NPO, the VT neurons show a ventro-dorsal gradient in the size of the neurons with smaller neurons occupying a ventral position and larger ones occupying a dorsal position (Figure 3a). The size difference was not apparent in the dorsally positioned IT neurons. Many pro-VT and pro-IT neurons occur in clusters forming contacts with their perikarya or processes especially in the NPO-pmc (Figures 3b and 3c). The IT neurons are clustered in large numbers than the VT neurons. While a large number of IT neurons cluster by perikaryal appositions (Figure 3c), fewer VT neurons form such cell body contacts (Figure 3a). However, a large number of VT neurons make contacts through their processes (Figure 3b). Due to perikaryal clustering, neurite contacts in IT neurons are not clear. The neurons belong to the bipolar, tripolar and multipolar types. The perikaryon is round, oval, triangular or club-shaped. The transcripts were mainly detected in the cell bodies and at the proximal part of the axons and dendrites (Figure 3b). Consequently, there was no staining of the axonal fiber tracts or their terminals in the neurohypophysis. The IT neurons can be seen interdigitating with the ependymal cells or lying in the ependyma (Figure 3d). The VT neurons though present around the preoptic recess was seen beneath the ependymal layer and no interdigitating neurons were observed.
Figure 3: Transverse sections of the brain through the brain of the catfish probed with the VT antisense probe (a) and (b), and IT antisense probe (c) and (d). The VT neurons are clubbed (arrows) and make neurite contacts (arrowheads). The IT neurons are heavily clustered than VT neurons (arrows) in (c). (d) IT neurons lying in the ependyma (arrows). V- ventricle, PR- preoptic recess.
Localization of pro-VT and pro-IT transcripts in the ovary
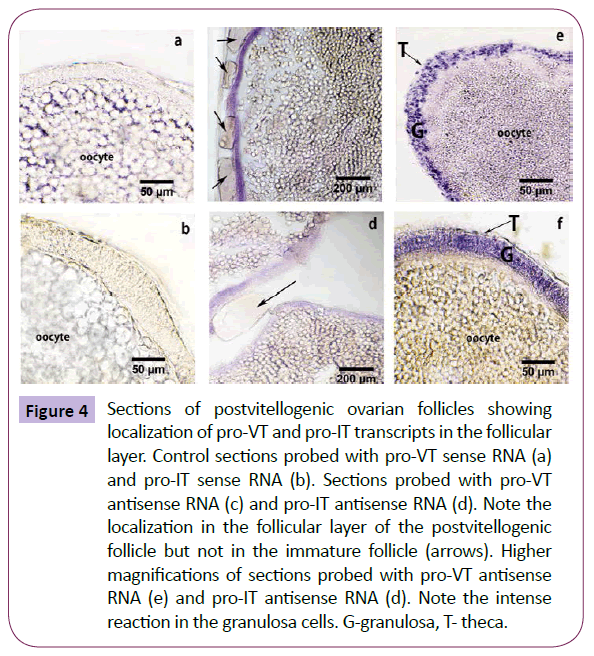
In the ovary, the hybridization with the sense probes did not yield any specific signal (Figures 4a and 4b), serving as a negative control. Pro-VT and pro-IT transcripts were localized in the follicular envelope of the post-vitellogenic oocytes, but not in the immature follicles (Figures 4c and 4d). The granulosa cells of the follicular layer showed intense reaction. Because of the scattered nature of the thecal cells, it was difficult to pin-point signals in the thecal cells at the resolution of the light microscope (Figures 4e and 4f).
Figure 4: Sections of postvitellogenic ovarian follicles showing localization of pro-VT and pro-IT transcripts in the follicular layer. Control sections probed with pro-VT sense RNA (a) and pro-IT sense RNA (b). Sections probed with pro-VT antisense RNA (c) and pro-IT antisense RNA (d). Note the localization in the follicular layer of the postvitellogenic follicle but not in the immature follicle (arrows). Higher magnifications of sections probed with pro-VT antisense RNA (e) and pro-IT antisense RNA (d). Note the intense reaction in the granulosa cells. G-granulosa, T- theca.
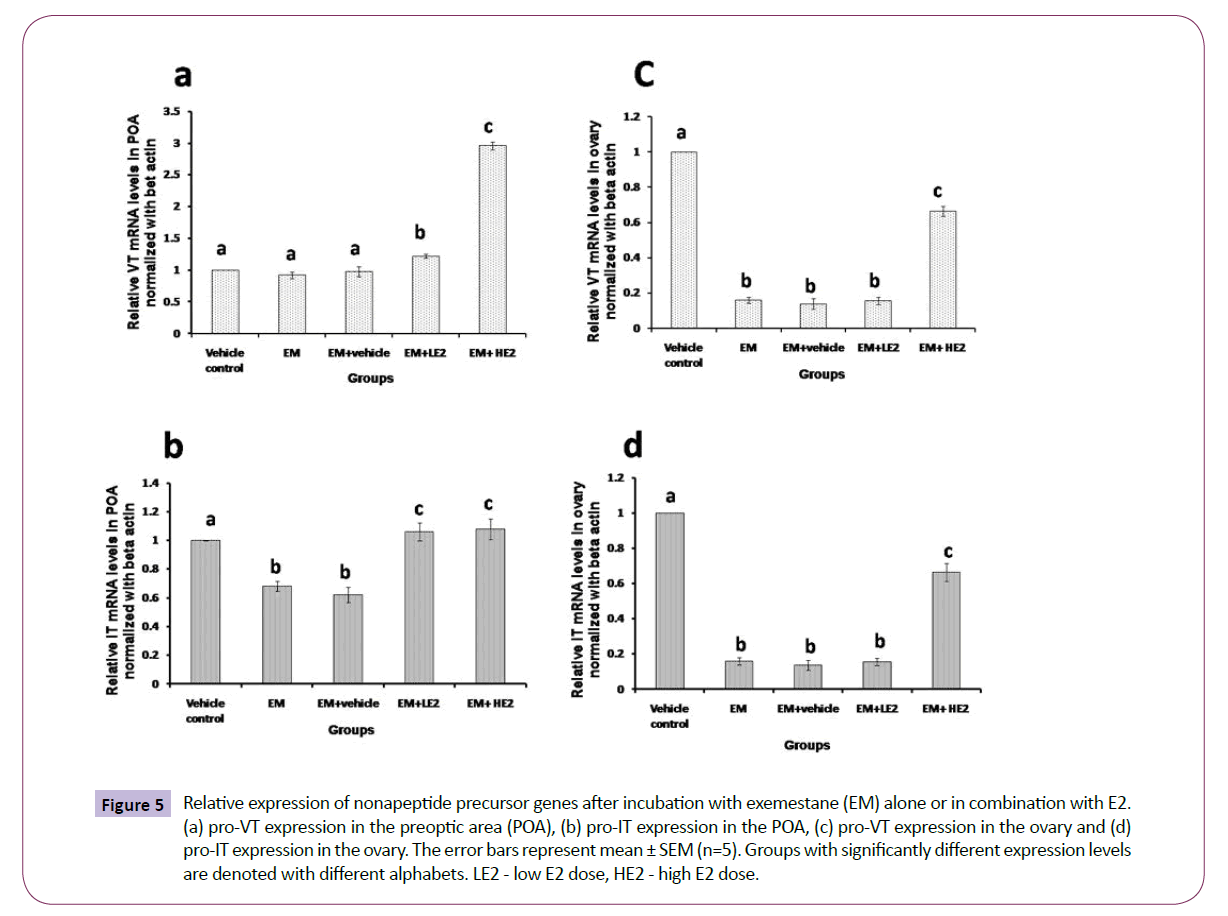
In vitro effects of exemestane and E2 supplementation on gene expression
There was an overall significant effect of the treatments with exemestane and E2 on the pro-VT gene transcription in the POA (Figure 5a; one way ANOVA, p < 0.001; F=396.29). The incubation of the POA with exemestane for 8 h did not alter the pro-VT transcript level. However, the co-incubation with low (1 nM) and high (10 nM) concentrations of E2 increased the transcript levels, the higher dose produced about a 3-fold increase.
Figure 5: Relative expression of nonapeptide precursor genes after incubation with exemestane (EM) alone or in combination with E2. (a) pro-VT expression in the preoptic area (POA), (b) pro-IT expression in the POA, (c) pro-VT expression in the ovary and (d) pro-IT expression in the ovary. The error bars represent mean ± SEM (n=5). Groups with significantly different expression levels are denoted with different alphabets. LE2 - low E2 dose, HE2 - high E2 dose.
The treatment with exemestane and E2 elicited overall significant effects on pro-IT precursor transcription in the POA (Figure 5b; one way ANOVA, p < 0.001; F= 4.66). The pro-IT transcript levels in the POA decreased after the exemestane treatment. The E2 supplementation restored as well as further increased the transcript levels significantly over that of the exemestane and control groups.
The incubations with exemestane and E2 produced overall significant effects (Figure 5c and 5d; one way ANOVA, p < 0.00) on the pro-VT (F= 1077.85) and pro-IT (F= 170.85) gene expression in the ovary. Exemestane drastically reduced the pro- VT and pro-IT gene expression (about 4-folds). The low dose of E2 supplementation did not restore the expression but the high dose could partially reverse the effect of exemstane but the levels were significantly lower than the basal levels.
Discussion
Expression of pro-VT and pro-IT in the brain: differential distribution and neuronal organization
This study reports the relative distribution of pro-VT and pro-IT neurons in the catfish brain. The VT neurons are concentrated in the antero-ventral part of the NPO-ppc to the caudo-dorsal part of the NPO-pmc. This pattern is similar to the distribution of immunoreactive VT neurons in the two subdivisions of the NPO, as reported previously [12]. In the catfish, the IT neurons were identified for the first time in this study. The IT neurons are present dorsally from the anterior to the posterior end. In the NPO of the related catfish C. batrachus, two clusters of immunoreactive IT neurons, one in the NPO-supraopticus located just above the optic chiasm and the other in the NPO-paraventricularis at the level of the third ventricle, were described [43]. In H. fossilis, we could not find the two distinct immunoreactive clusters and the pro-IT distribution may correspond to that of the NPO-paraventricularis of C. batrachus. The distribution pattern of VT and IT neurons varies with teleosts. In sea bream [44], rainbow trout [45] and zebrafish [46], the VT and IT neurons have differential spatial distribution within the NPO like H. fossilis. On the other hand in goldfish, medaka, European plaice, green molly and false clown anemonefish, VT and IT neurons are evenly distributed in the NPO [47-50].
In addition to the NPO, a few neurons in the anterior division of the NLT in the ventral hypothalamus showed pro-VT-positive signal. However, no immunoreactive VT neurons were described in the NLT region previously [12]. The presence of VT/IT neurons in centers other than NPO has been reported in plainfin midshipman [51,52]. In medaka, VT neuron populations were described additionally in NPT (nucleus posterior tuberis), aNVT (anterior part of nucleus ventral tuberis), NAT (nucleus anterior tuberis) and pNVT (posterior part of nucleus ventral tuberis) in the hypothalamus, some of which are sexually dimorphic [50]. In larval zebrafish, in situ hybridization showed two domains of pro-VT gene expression, the dorsal preoptic area and the ventral hypothalamus. However, VT-positive neurons were not detected immunohistochemically in the hypothalamus of both adult and larval zebrafish neurons [46]. Since this study in zebrafish indicates that presence of transcript may not necessarily mean the presence of peptide, in the catfish too there is a need to analyze the functional implication of VT transcript expression in the NLT at the peptide level.
In the NPO, the VT neurons displayed a ventro-dorsal gradient in their size. The gradient in the neuron size has been attributed to physiological regeneration by endoreplication to meet increased demands during periods of heightened activity like spawning and migration [53,54]. The large neurons present in the dorsal side may be the ones that have undergone endoreplication that leads to an increase in cell and nuclear size. Desai and Akhunji [55] reported binucleated neurons and neurons having horseshoeshaped nuclei in the NPO of the teleost Pampus argenteus, which indicates that a genome amplifying mechanism may be operative in the magnocellular neurons. These neurons may cater to very high secretory demands. In the present study, a gradient in neuron size was observed only for the VT neurons, and not for the IT neurons, and may indicate that the gene amplifying mechanism may be operative only in the VT neurons. Apart from a device to fulfill increased demands, endoreplication may also provide the advantage that a single large neuron would have, over several smaller ones, the advantage in warding off the need of a complex coordinating system [56].
The neuron-specific clusters that were observed for IT neurons also indicate that mechanisms leading to a high degree of coordination in neurons producing the same peptide are highly favored in the NPO. Saito et al. (2004) have reported neuronspecific clusters in the rainbow trout NPO. Cumming et al. [57] reported direct soma to soma appositions without intervening glial sheath in the goldfish NPO cells. Such contacts may facilitate communications by locally changing the membrane potential or release of the peptides [58]. The IT neuron clustering in the NPO of the catfish may be a physiological adaptation for neuron- toneuron communications leading to a high degree of coordination. Apart from perikaryal clustering, the NPO neurons make contacts through their processes. In the catfish such contacts are visibly high among VT neurons as compared to the IT neurons. In the rainbow trout, close appositions of VT proximal processes were described [45]. Cumming et al. [57] reported axo-dendritic synapses with VT-immunoreactive axon terminals in pars magnocellularis, facilitating a communication between the VT neurons. Such contacts among the IT neurons are less frequent in the rainbow trout [45], which is similar to the case in the catfish. The localization study in the brain shows that both VT and IT neurons have a discrete distribution in the NPO with distinct spatial and neuronal organization, implying distinct physiological functions and regulations.
Expression of pro-VT and pro-IT in the ovary
Both pro-VT and pro-IT transcripts are distributed in the follicular layer of the oocytes. The pro-VT transcript localization in the ovary supports an earlier observation of VT immunoreactivity in the theca and granulosa cells of the ovarian follicles [12] and the study confirms that VT is synthesized de novo in the ovary. The study also reports the localization of pro- IT for the first time in the follicular layer. Further, both pro-VT and pro-IT transcripts were observed only in the fully grown post-vitellogenic follicles and not in the immature follicles. The study corroborates an earlier study by qRT-PCR in which the pro-VT and pro-IT expression was elicited by the isolated follicular envelope, and not in the denuded oocytes. The transcript levels in the ovary was shown to increase with gonadal recrudescence, reaching the peak in the spawning phase [24] when the catfish ovary contains the highest percentage of post-vitellogenic follicles. The nonneuronal expression of pro-VT and pro-IT in the ovary implies de novo production of the hormones in the ovary where they may act as paracrine/autocrine factors. Earlier studies from our laboratory have shown that VT has important physiological roles such as stimulation of steroidogenesis, prostaglandin secretion, oocyte hydration, final oocyte maturation (FOM) and ovulation [13-16,35]. Further, the expression of VT receptor genes (v1a1, v1a2 and v2A) was demonstrated in the catfish ovary [19]. Taken together these studies show that the catfish ovary has a functional VT system where it acts as a paracrine/autocrine factor. Although IT was found to be less potent in these functions [13-14], the presence of pro-IT in the follicular envelope like pro- VT indicate a paracrine role of IT too, but needs further study. In rainbow trout, IT gene expression was higher than VT expression during oocyte maturation [23]. The physiological relevance of extra-neural expression of nonapeptides may necessitates additional regulatory mechanisms that can cater exclusively to ovary-specific requirements. Therefore, the estrogen regulation of the nonapeptide precursor genes in the brain and ovary was separately investigated.
Estrogen regulation of expression of pro-VT and pro-IT in the brain and ovary
In teleost ovary, estradio-17β (E2) is the principal estrogen and is mainly concerned with the induction of hepatic vitellogenin synthesis and feedback regulation of gonadotropins. Estrogens are also produced in the brain and are concerned with brain functions such as sexual differentiation, neuronal proliferation and growth, behaviour, etc. [32]. In the catfish, both brain- and ovary-specific aromatase genes and E2 were demonstrated [33,35]. Therefore, in the present study we employed a different strategy to differentiate the effects of E2 derived from the ovary and brain on the nonapeptide gene expression in the NPOcontaining POA and ovarian follicles In vitro. The earlier studies using ovariectomy and E2 replacement model could show only the role of ovary-derived estrogen on nonapeptide secretion. Indeed, ovariectomy decreased, and E2 replacement reversed the effect of ovariectomy depending on the steroid dose [30,59]. The POA is a major site of aromatase activity and neuroestrogen production [34]. Exemestane is a known inhibitor of Cyp19a1a (ovarian form). The concentration used in this experiment was about 100 times more than the IC50 value for cyp19a1a. It is very likely that the brain isoform Cyp19a1b may also be inhibited at this concentration causing a significant reduction in the total pool of estrogen in the brain and ovary. From the data, it is evident that the POA experimental model has responded to the exemestane and exogenous E2 treatments. The exemstane treatment did not alter the pro-VT gene expression but inhibited the expression of pro-IT. In other words, pro-VT expression may not be influenced by the POA-derived estrogen but the pro-IT expression seems to be modulated by the endogenous E2. The results suggest that VT and IT synthesized in distinct neurons in the NPO are differently controlled by the locally produced E2. In the catfish, it is not known, if VT and IT neurons synthesize neuroestrogens though the POA is aromatase- rich [34]. On the other hand, it is likely that estrogen receptor subtypes (ER-α, ER-βa or ER- βb) are distributed in different divisions of the NPO containing the VT and IT neurons [36,37]. In bluehead wrasse, aromataseimmunoreactive fibers (radial glial cells) are closely associated with VT-immunoreactive neurons throughout the POA, indicating the potential for functional interactions [60]. In the catfish, radial glial cells in the telencephalon and preoptic recess showed strong signals for brain type aromatase gene [35]. Both pro-VT and pro- IT expression increased in co-incubations of exemstane and E2. While the VT expression responded strongly to the high E2 dose (10 nM), the magnitude of IT expression was low but both doses responded equally. The results suggest that the VT and IT neurons respond to the exogenously supplemented/circulating peripheral estrogens and the IT neurons may be highly sensitive than the VT neurons. The previous ovariectomy and E2 replacement studies in the catfish reiterate the results. The differential dose effects of E2 and the lack of response of the VT expression to the exemstane treatment may be due to differential distribution and sensitivity of the ERs. The IT neurons may be rich in ERs or more sensitive to estrogen regulation than the VT expression. Further work is needed for a direct evidence of nonapeptide gene expression and ER interactions.
In the ovary, exemestane drastically reduced the pro-VT and pro-IT gene expression, implying that the ovarian E2 modulates the basal nonapeptide gene expression. The low dose of E2 did not restore the expression but the high dose did. It is possible that the 1 nM dose may not be sufficient to reverse the effect of exemstane and increase the gene expression. Our previous study reports that E2 exerts biphasic effects (stimulatory or inhibitory) depending on the dose, duration and reproductive phase [30]. In the preparatory phase, low doses (1 ng/mL and 10 ng/mL) were stimulatory and high dose (100 ng/mL) was inhibitory but in the prespawning phase, these doses were all inhibitory in a dosedependent manner. Exemstane is strongly effective to decrease the gene expression in the ovary than the POA. This may be due to the fact E2 acts in a paracrine/autocrine manner in the ovarian follicles. Both E2 and nonapeptide genes are localized in the granulosa cells [35]. In the POA, E2 may act through the receptors directly or indirectly through other neural inputs like catecholamines [18,33]. The POA (NPO) nonapeptide systems are exposed to multiple control mechanisms since the hormones are secreted into the blood for the regulation of multiple physiological functions such as osmoregulation, stress, metabolism, reproduction, behaviour, circadian rhythms, and cardiovascular functions [3]. Therefore, the expression of nonapeptide genes in the NPO is regulated and integrated by various peripheral inputs. This is in contrast to the regulation of the nonapeptide genes in the ovary, which execute only the ovary-specific functions.
In conclusion, the present study demonstrates the spatial distribution of the nonapeptide precursor transcripts in the brain and ovary, and the differences in the extent of regulatory influence of E2 on the expression of the nonapeptide precursor genes in both the sites. This study forms a basis for future studies involving estrogen receptors and promoter activity of the genes.
Acknowledgment
The research work was supported by a research grant of Department of Science and Technology, New Delhi (Grant No. SA/ SO/AS-43/2009) to KPJ and RC. The first author is grateful to the Council of Scientific and Industrial Research, New Delhi for award of Junior and Senior Research Fellowships.
References
- Acher R (1996) Molecular evolution of fish neurohypophysial hormone: neutral and selective evolutionary mechanism. Gen Comp Endocrinol 102: 157-172.
- Urano A, Kubokawa K, Hiraoka S (1994) Expression of the vasotocin and isotocin gene family in fish. In: Sherwood NM, Hew CL (eds), ‘‘Fish Physiology’’Academic Press, New York, 13: 101-132.
- Balment RJ, Lu EW, Weybourne W, Warne JM (2006) Arginine vasotocin: a key hormone in fish physiology and behaviour: A review with insights from mammalian models. Gen Comp Endocrinol 147: 9-16.
- Gimpl G, Fahrenholz F (2001) The oxytocin receptor system: structure, function, and regulation. Physiol Rev 81: 629-83.
- Wathes DC, Swann RW, Birkett SD, Porter DG, Pickering BT (1983) Characterization of oxytocin, vasopressin and neurophysin from bovine corpus luteum. Endocrinology 113: 693-698.
- Nicholson HD, Swann RW,Burford GD, Wathes DC, Porter DG, Pickering BT (1984) Identification of oxytocin and vasopressin in the testis and in adrenal tissue.RegulPept 8: 141-146.
- Nussey SS, AngnVTY, Jenkins JS, Chowdrey HS, Bisset GW (1984) Brattleboro rat adrenal contains vasopressin. Nature 310: 64.
- Schaeffer JM, Liu J, Hseuh AJW, Yen SSC (1984) Presence of oxytocin and arginine vasopressin in human ovary, oviduct and follicular fluid. J ClinEndocrinolMetab 59: 970-973.
- Geenen V, Legros JJ, Franchimont P,Baudrihaye M, Defresne MP,Boniver J (1986) The neuroendocrine thymus: coexistence of oxytocin and neurophysin in the human thymus, Science232: 508-511.
- Kasson BG,Adashi EY,Hsueh AJ(1986) Arginine vasopressin as an intragonadal hormone in Brattleboro rats: presence of a testicular vasopressin-like peptide and functional vasopressin receptors. Endocrinology 118: 23-31.
- Saito N, Kinzler S, Koike TI (1990) Arginine vasotocin and mesotocin levels in the theca and granulosa layers of ovary during the oviposition cycle in hens (Gallus domesticus). Gen Comp Endocrinol 79: 54-63.
- Singh V, Joy KP (2008)Immunocytochemical localization, HPLC characterization and seasonal dynamics of vasotocin in the brain, blood plasma and gonads of the catfish Heteropneustesfossilis. Gen CompEndocrinol 159: 214- 225.
- Singh V, Joy KP (2009) Relative In vitro seasonal effects of vasotocin and isotocin on ovarian steroid hormone levels in the catfish Heteropneustesfossilis. Gen Comp Endocrinol 162: 257- 264.
- Singh V, Joy KP (2010) An involvement of vasotocin in oocyte hydration in the catfish Heteropneustesfossilis: A comparison with effects of isotocin and hCG. Gen Comp Endocrinol 166: 504- 512.
- Singh V, Joy KP (2011)Vasotocininduces finaloocyte maturation and ovulation through the production of a maturation-inducing steroidin the catfish Heteropneustesfossilis. Gen CompEndocrinol 174:15- 21.
- Joy KP,Singh V (2013) Functional interactions between vasotocin and prostaglandins during final oocyte maturation and ovulation in the catfish Heteropneustesfossilis. Gen Comp Endocrinol 186:126-135.
- Joy KP, Chaube R (2015)Vasotocin- A new player in the control of oocyte maturation and ovulation in fish. Gen Comp Endocrinol 221: 54-63.
- Singh R K, Chaube R, Joy KP (2013) Differential and reproductive stage-dependent regulation of vasotocin secretion by catecholamines in the catfish Heteropneustesfossilis. CompBiochemPhysiol Part A 166: 619-626.
- Rawat A, Chaube R, Joy KP (2015) Molecular cloning, sequencing and phylogeny of vasotocin receptor genes in the air-breathing catfish Heteropneustesfossilis with sex dimorphic and seasonal variations in tissue expression. Fish PhysiolBiochem 41: 509-532.
- Ramallo MR,GroberM,Cánepa MM, MorandiniL, PandolfiM (2012) Arginine-vasotocin expression and participation in reproduction and social behavior in males of the cichlid fish Cichlasomadimerus. Gen Comp Endocrinol179: 221-231.
- Rodríguez M, Specker JL (1991). In vitro effects of arginine vasotocin on testosterone production by testes of rainbow trout (Oncorhynchusmykiss). Gen Comp Endocrinol.83: 249-257.
- Pickford GE,Strecker EL (1977). The spawning reflex response of the killifish, Fundulusheteroclitus: Isotocin is relatively less active in comparison with argininevasotocin. Gen Comp Endocrinol 32: 132-137.
- Bobe J, Montfort, J, Nguyen T, Fostier A, (2006). Identification of new participants in the rainbow trout (Onchorhynchusmykiss) oocyte maturation and ovulation process using cDNA microarrays. ReprodBiolEndocrinol 4: 39-54.
- Banerjee P, Chaube R and Joy KP (2015) Molecular cloning, sequencing and tissue expression of vasotocin and isotocin precursor genes from Ostariophysian catfishes: phylogeny and evolutionary considerations in teleosts. Front Neurosci 9:166.
- Voorhuis TA M, Kiss JZ, de Kloet ER, de Wied D (1988) Testosterone sensitive vasotocin-immunoreactive cells and fibers in the canary brain. Brain Res 442:139–146.
- Adan RAH, Burbach JPH, (1992) Regulation of vasopressin and oxytocin gene expression by estrogen and thyroid hormones. ProgBrain Res932: 127-136.
- Miller MA, De Vries GJ,Hussien A, Shamma Al,Dorsa MD (1992) Decline of vasopressin immunoreactivity and mRNA levels in the bed nucleus of striaterminalis following castration.JNeuroendicrinol12: 2281-2887.
- Moore Fl, Wood R, Boyd SK (1992) Sex steroids and vasotocin interact in a female amphibian (Tarichagranulosa) to elicit female like egg laying or male like courtship. HormBehav 13: 207-213.
- Boyd SK (1994) Gonadal steroid modulation of vasotocin concentrations in the bullfrog brain. Neuroendocrinology 60: 150-156.
- Singh V, Joy KP (2009b) Effects of hCG and ovarian steroid hormones on vasotocin levels in the female catfish Heteropneustesfossilis. Gen Comp Endocrinol 162: 172-178.
- Ota Y, Ueda H, and Urano A (1996). Seasonal changes in expression of neurohypophysial hormone genes in the preoptic nucleus of masu salmon. Gen CompEndocrinol116:31-39.
- Ota Y, Ando H, Ueda H, Urano A (1999). Differences in seasonal expression of neurohypophyseal hormone genes in ordinary and precocious male masu salmon. Gen Comp Endocrinol 116: 40-48.
- Chaube R,Mishra S (2012) Brain steroid contents in the catfish Heteropneustesfossilis: Sex and gonad stage-specific changes. Fish PhysiolBiochem 38: 757-767.
- Diotel N, Page YL, MauriecK, Tong SK, Pellegrini E, et al. (2010) Aromatase in the brain of teleost fish: Expression, regulation and putative functions. Front. Neuroendocrin31: 172-192.
- Chaube R, Rawat A, JoyKP (2015) Molecular cloning and characterization of brain and ovarian cytochrome P450 aromatase genes in the catfish Heteropneustesfossilis: Sex, tissue and seasonal variation in, and effects of gonadotropin on gene expression, Gen Comp Endocrinol221: 120-133.
- Hawkins MB, Godwin J, Crews D, Thomas P (2005)The distributions of the duplicate oestrogen receptors ER-βa and ER-βb in the forebrain of the Atlantic croaker (Micropogoniasundulatus): evidence for subfunctionalization after gene duplication ProcBiolSci 272: 633–641.
- MuriachB, Cerdá-ReverterJM, Gómez A, ZanuyS, Carrillo M (2008). Molecular characterization and central distribution of the estradiol receptor alpha (ERα) in the sea bass (Dicentrarchuslabrax) . J ChemNeuroanat 35: 33-48.
- Di SE,Ornati G,Giudici D,Lassus M,Evans TR, et al. (1992). Exemestane (FCE 24304), a new steroidal aromatase inhibitor. J Steroid BiochemMolBiol43: 137-143.
- Lagendijk AK, Moulton JD, Bakkers J(2012) Revealing details: whole mount microRNA in situ hybridization protocol for zebrafish embryos and adult tissues. Biol Open.
- Forsgren KL, Young G (2012) Specific effects of androgen and estradiol 17β on the development of late primary and early secondary ovarian follicles of Coho Salmon (OnchorhymchusKisutch) In vitro. BiolReprod87: 1-14.
- Soudon J (2000) Comparison of In vitro exemestane activity versus other antiaromatase agents, Clinical Breast Cancer 1: 68-73.
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCT method. Methods 25: 402–408.
- Singh U, Kumar S, Singru PS (2012) Interaction between dopamine- and isotocin-containing neurones in the preoptic area of the catfish,Clariasbatrachus: Role in the regulationofluteinising hormone cells. JNeuroendocrinol 24: 1398–1411.
- Duarte G, Segura-Nogvera MM,Martin del Rio MP,Mancera JM (2001) The hypothalamo-hypophyseal system of the white sea bream Diplodussargus: immunocytochemical identification of arginine-vasotocin, isotocin, melanin-concentrating hormone and corticotrophin releasing factor. HistochemJ33: 569–578.
- Saito D, Komatsuda M, Urano A (2004) Functional organization of preopticvasotocin and isotocin neurons in the brain of rainbow trout: central and neurohypophysial projections of single neurons. Neurosci 124: 973–984.
- Herget U, Wolf A, Wullimann MF, Ryu S (2014) Molecular neuroanatomy and chemoarchitecture of the neurosecretorypreoptic-hypothalamic area in zebrafish larvae. J CompNeurol 522:1542–1564.
- Goossens N, Dierickx K, Vandesande F (1977)Immunocytochemical localization of vasotocin and isotocin in the preoptico-hypophysialneurosecretory system of teleosts. Gen Comp Endocrinol 32: 371–375.
- Batten TFC, Cambre ML, Moons L, Vandesande F (1990) Comparative distribution of neuropeptides- immunoreactive systems in the brain of the green molly (Poecilialatipinna). J Comp Neurol 302:893–919.
- Iwata E, Nagai Y, Sasaki H (2010) Immunohistochemistry of brain arginine vasotocin and isotocin in false clown anemonefishAmphiprionocellaris. The Open Fish Science Journal 3:147-153.
- Kawabata Y,Hiraki T,Takeuchi A, Okubo K(2012) Sex differences in the expression of vasotocin/isotocin, gonadotropin-releasing hormone, and tyrosine and tryptophan hydroxylase family genes in the medaka brain. Neurosci218: 65-77.
- Goodson JL, Bass AH (2000) Vasotocin innervations and modulation of vocal- acoustic circuitry in the teleost Porichthysnotatus.J CompNeurol 422: 363-379.
- Goodson JL, Evans AK, Bass AH (2003). Putative isotocin distribution in sonic fish: relationship to vasotocin and vocal acoustic circuitry. J Comp Neurol462: 1-14.
- Polenov AL,Chetverukhin VK, (1993) Ultrastructuralradioautographic analysis of neurogenesis in the hypothalamus of the adult frog, Ranatemporaria, with special reference to physiological regeneration. Cell Tiss Res 271: 351-362.
- Knobloch HS, Grinevich V (2014) Evolution of oxytocin pathways in the brain of vertebrates. Front BehavNeurosci.
- Desai K, AkhunjiUU (1971) Histological studies on the hypothalamo- neurohypophyseal complex ofPampusargenteus(Euphr). AnnotZoolJapon 44:161-169.
- Mandriorli M, Mola L, Coughi B, Sonetti D (2010)Endoreplication: A molecular trick during animal neuron evolution. Q Rev Biol 85: 159-169.
- Cumming R, Reaves TA, Jr. Hayward JN (1982) Ultrastructuralimmunocytochemical characterization of isotocin, vasotocin and neurophysin neurons in the magnocellular pre-optic nucleus of goldfish. Cell Tiss Res223: 685-694.
- Ludwig M, Sabatier N, Bull PM, Landgorf R, Dayanithi G, Leng G (2002) Intracellular calcium stores regulate activity-dependent neuropeptide release from dendrites. Nature 418: 85-89.
- Chaube R, Singh RK, Joy KP (2012) Estrogen regulation of brain vasotocin secretion in the catfish Heteropneustesfossilis: an interaction with catecholaminergic system. Gen Comp Endocrinol 175: 206-213.
- Marsh KE, Creutz LM, HawkinsMB, Godwin J (2006)Aromatase immunoreactivity in the bluehead wrasse brain, Thalassomabifasciatum: Immunolocalization and co-regionalization with arginine vasotocin and tyrosine hydroxylase. Brain res 1126: 91-101.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences