In vitro Influence of Different Fatty Acids on the Pharmacological Effect of Temozolomide
Yehoshua Maor*, Marcel Benadiba , Osnat AlmogiHazan , Raphael Serruya and Reuven Or
DOI10.21767/2573-5349.100021
Yehoshua Maor1*, Marcel Benadiba1, Osnat Almogi- Hazan2, Raphael Serruya1 and Reuven Or2
1Phytor Lab for Drug Development, The Hadassah Medical Center, Hebrew University Biotechnology Park (JBP), Ein Kerem Campus, Jerusalem 91120, Israel
2Department of Bone Marrow Transplantation, Hadassah University Hospital, Ein Kerem Campus, Jerusalem 91120, Israel
- *Corresponding Author:
- Yehoshua Maor
Phytor Lab for Drug Development
The Hadassah Medical Center
Hebrew University Biotechnology Park (JBP)
Ein Kerem Campus, Jerusalem 91120, Israel
Tel: +972 58 414 9443/ +972 2502 5229
E-mail: yehoshua.maor@mail.huji.ac.il
Received Date: July 10, 2018; Accepted Date: July 23, 2018; Published Date: July 27, 2018
Citation: Maor Y, Benadiba M, Almogi- Hazan O, Serruya R, Or R (2018) In vitro Influence of Different Fatty Acids on the Pharmacological Effect of Temozolomide. J Transl Neurosci 3:8. DOI: 10.21767/2573-5349.100021
Abstract
Introduction: Glioblastoma multiforme is the most malignant cancer in the human body with limited treatment options, in which Temozolomide (TMZ) is the conventional chemotherapeutic agent (CA) often prescribed for this condition. Fatty acids (FAs) per se, including polyunsaturated fatty acids (PUFAs), have been prescribed as adjuvants molecules for general cancer treatment. However, little is known about potential interactions between these lipids and the CAs. TMZ was combined with different FAs to access the nature of the interactions between these compounds.
Methods: Two in vitro barrier models of the gut-brain axis (GBA) and the blood-brain barrier (BBB) were used. In each of them, 2-hydroxyoleic acid (2-OHOA); gamma-linolenic acid (GLA); and fish oil (FO) which contains both docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), were used as treatments.
Results: In both GBA and BBB models, the combination of TMZ with each FA elicited antagonistic effects, interfering with the transepithelial electrical resistance (TEER) and producing less or no response in the inhibition of T98G cell proliferation. This was associated with alterations in cell morphology, β-catenin and E-cadherin protein expression, and induction of lipid droplets.
Conclusion: Using our in vitro barrier models, we concluded that the FA might negatively interfere with TMZ`s cytotoxic effect. On the other hand, TMZ also may play a negative role in the antitumor action of specific FA like GLA or 2-OHOA, as observed in the GBA model. These results call into question the clinical utilization of these FA as nutritional adjuvants for patients under the use of TMZ.
Keywords
Glioblastoma multiforme; Temozolomide; 2-hydroxyoleic acid; Fatty acids; Gut-brain axis; Blood-brain barrier
Introduction
Gliomas are Glial cell-derived primary, central nervous system (CNS) brain tumors, of which Glioblastoma multiforme (GBM) appears as the most malignant and aggressive form [1-3]. GBM is characterized by its fast and devastating growth on brain tissue. Complaints among patients may vary from headaches, nausea, fainting, seizures, etc., which usually start when the tumor has grown into such proportions that the intracranial pressure is high [4]. At this stage, treatment options are scarce, mainly involving surgical resection of the tumor, followed by radiotherapy and chemotherapy [5]. However, its highly proliferative and invasive nature along with its localization in the brain (i.e., cortex, brain stem, basal ganglia, etc.) render this type of cancer very difficult to treat [6]. Additionally, the residual cancer cells that remain after surgical resection, located on the borders of the tumor, have the potential to cause cancer to recur within a period of six to nine months [7]. Patient survival after diagnosis with GBM is usually about 12-18 months [8].
Temozolomide (TMZ) is currently the standard chemotherapeutic agent (CA) for newly diagnosed GBM and is usually combined with radiation therapy to improve therapeutic outcomes [6,9]. Studies have also tested the combination of TMZ with other CAs; however, no improved clinical benefits were observed [6,10]. Due to the unresponsiveness (specifically in GBM with hypo methylated MGMT promoters), intrinsic resistance and the role of the blood-brain barrier (BBB) in preventing access of the cytotoxic agents to the tumor, the search for new therapies with low toxicity profiles continues [11-13]. Thus, to the best of our knowledge, TMZ combined with radiotherapy remains the single proven therapy able to slightly, yet significantly, increase patient survival, albeit with reduced or no significant improvement in their quality of life due to its side effects [11,14-16].
Over the past decade, lipid molecules or fatty acids (FA) have attracted attention as anticancer agents. A synthetic FA, known as 2-Hydroxyoleic acid (2-OHOA), has recently entered phase IIB clinical trials for patients with newly diagnosed malignant glioma [17]. 2-OHOA is thought to mediate its anti-tumor effect by affecting the biophysical properties of membranes, which leads to altered recruitment and activation of amphitropic proteins, altered cellular signaling, and eventual cell death [18- 20]. 2-OHOA is a synthetic analog of oleic acid and is believed to have a slower metabolism, a longer half-life, and thus a long-lasting pharmacological effect [19]. The phase IIB study with this compound aims at assessing the potential clinical benefit of adding 2-OHOA to the current standard protocol (i.e., TMZ and radiotherapy) in patients with newly diagnosed GBM [17].
Natural products have recently emerged as compelling tools for improving TMZ efficiency, as well as reducing its side effects [21,22]. For instance, the addition of Epigallocatechin gallate (EGCG), a phenolic compound of green tea, has shown promising results in preclinical studies [23]. Naturally occurring lipid molecules or fatty acids (FA) derived from plants or animals have been tested for anticancer activity [24]. Docosahexaenoic (DHA) and eicosapentaenoic (EPA) acids are both omega-3 FA from fish oil (FISH). They are considered potential protective agents against several types of cancers, including breast, prostate and colorectal cancer, but not for brain cancer [24-26]. Conversely, γ-linolenic acid (GLA), an omega-6 FA highly present in primrose and borage oils, is the most studied FA in human gliomas [27,28]. Clinical and pre-clinical studies have already demonstrated its ability to arrest tumor growth by inducing apoptosis, as well as decreasing cell proliferation, invasion and angiogenesis [28-30]. Previous studies showed that GLA has significant specificity for tumor cells [30-33].
The effects of the abovementioned FA (Figure 1A) in combination with TMZ has not been investigated so far. Thus, in this study we analyzed the effect of combining TMZ with different FAs. For that, we used the gut-brain axis (GBA) and the BBB axis in vitro models translationally significant to delve into the mechanism of pharmacological interaction and absorption in the gut with subsequent effect in the brain (Figure 1B).
Figure 1: A) Molecular structures of fatty acids, Eicosapentaenoic acid (EPA), Docosahexaenoic acid (DHA), γ-linolenic acid (GLA), α-linolenic acid (ALA) and 2-Hydroxyoleic acid (2-OHOA). Modified from PubChem [42]. B) Schematic representation of the GBA, gut-brain axis (left) and the BBB, blood-brain barrier (right) in vitro models.
Materials and Methods
Compounds
2-Hydroxyoleic acid (2-OHOA) was purchased from Avanti Plar Lipids Inc., Alabama, USA. Gamma-Linolenic acid (GLA) was purchased from Sigma® (cat. L2378). Fish oil (FISH) was obtained from Sigma® (cat. 8002-50-4).
Cell culture
Human glioblastoma (T98G) cells, colon cancer (Caco-2) cells, and human umbilical vein endothelial cells (HUVEC) were cultured according to standard, mammalian, tissue culture protocols and sterile conditions. T98G was cultured in RPMI 1640 medium and Caco-2 in high glucose, Dulbecco’s Modified Eagle Medium (DMEM), which were supplemented with 10% fetal bovine serum (FBS). HUVEC cells were cultured in Endothelial Cell Growth Medium 2 supplemented with SupplementMix C-39216. A combination of streptomycin (100 μg/ml), penicillin (100 U/ ml) and nystatin (12.5 U/ml) were used as antibiotics. Incubation conditions were 5% CO2 atmosphere at 37°C. All tissue cultures were maintained in 25 cm2 NuncTM cell culture treated EasYFlaskTM (Thermo Fisher Scientific). RPMI and DMEM mediums, as well as FBS and antibiotics, were obtained from Biological Industries®. The HUVEC medium and its supplement were purchased from PromoCell®.
MTT assay
The viability of the cells following treatment was determined using a commercially available MTT assay kit (ABCAM, ab146345) and performed according to manufacturer instructions. Briefly, cells were seeded in 96-well plates at a density of 1 × 104 cells/well (n=4). After overnight stabilization, cells were exposed to varying concentrations of 2-OHOA, GLA, FISH, and TMZ. Based on the literature, the concentration range was 25-400 μM for GLA and 2-OHOA, 3-100 μg/ml for FISH (a second experiment was performed using 3-50 mg/ ml), and 0.25-500 μM for TMZ. The EC50 was obtained for each compound to be used in the dual chamber model of cell cultures. Plates were incubated in a humidified atmosphere containing 5% CO2 in air at 37°C for 24 hours. According to the MTT standard protocol, after the treatment period, the media was removed, and all cells were incubated with serum-free media containing 0.5 mg/ml MTT for four hours in the incubator. The MTT purple crystals formed by the viable cells were diluted using isopropanol containing 0.04 mol/L HCl. The quantification was determined by measuring the optical density at 570 nm in an enzyme-linked immunosorbent assay (Spark, Tecan) reader. Data were presented as proportional viability (%) by comparing the treated group with the untreated cells, the viability of which is assumed to be 100%.
Transepithelial Electrical Resistance (TEER)
Caco-2 or HUVEC were plated in a 6.5 mm Transwell with 0.4 μm pore polycarbonate membrane insert (Millipore®). T98G cells were plated in the basolateral side on the bottom of the well in a 24-well polystyrene plate Corning® Costar®. For a more realistic development of the BBB in vitro, human astrocyte-derived cells were plated upside down on the surface of each insert (Figure 1B). Inserts were incubated for one hour with 0.01% Poly-L-lysine solution (P4707 - Sigma®) prior to seeding, to facilitate cellular attachment. After 72 hours of seeding, the treatment was set for 24 hours. TEER (Transepithelial electrical resistance) was used to determine the monolayer integrity. At the end of the treatment, plates were equilibrated at room temperature for one hour. The electrical resistance (ER) across the monolayer was measured using an Ohm meter (Millicell®) equipped with probes, positioning the probes one inside the filter well and the second into the medium in the growth well. The electrical resistance for each well was recorded. The ER of a blank insert (without cells) was determined to subtract the background value.
Trypan blue exclusion assay
The number of viable cells in each well of the 24-well plate described above and in 25 cm2 flasks was determined by exclusion of 0.1% w/v trypan blue dye (Sigma®), and cells were counted with a hemocytometer. The trypan blue exclusion method was also used to seed viable cells.
Immunoblot analysis
Immunoblot analysis was performed using antibodies against E-cadherin (1:10000 dilution; ab40772, Abcam), β-catenin (1:1000 dilution; ab227499, Abcam), and the internal control β-actin (1:1000 dilution; ab101173, Abcam). Species-specific HRP-labeled secondary antibodies were used. The blots were visualized using enhanced chemiluminescence (Biological Industries) and quantitated using ImageQuant LAS 4000 mini, General Electric.
Cell morphology and imaging analysis
All imaging was performed using a Zeiss Axiocam 105 color in a Carl Zeiss™ Primo Vert™ inverted microscope with the Zen Pro software suite. Phase-contrast images were acquired with 10x, 20x and 40x objective lenses. To quantify T98G cell proliferation, cells were counted after 24 h treatment and compared with the images. To analyze images (40x magnification) in ImageJ software (https://imagej.nih.gov/ij/), cell area was acquired and compared. The thickness of the intercellular space was also measured using Pixcavator Image Analysis software (https://inperc.com/image_analysis.htm).
Nile red staining
Following treatment, cells were fixed with 4% formaldehyde for 20 minutes at room temperature and washed 3 times with 1X PBS. To stain with Nile Red (Sigma®), 2 μL of a 1 mg/mL stock solution was added to 20 mL of 150 mM NaCl in PBS to make a Nile Red solution. The Nile Red solution was added to the cells and incubated for 20 minutes in the dark. Following incubation, the cells were washed 3 times with 1X PBS and stained with 5 μg/ml Hoechst 33342 (Sigma®) for more 20 minutes. Cells were imaged on an ECLIPSE Ts2-FL (Diascopic and Epi-fluorescence illumination model) Microscope. Images were processed using ImageJ software.
Statistical analysis
We used Student’s t-test for statistical analysis on Microsoft Excel. Data were considered significantly different from control when p<0.05.
Results
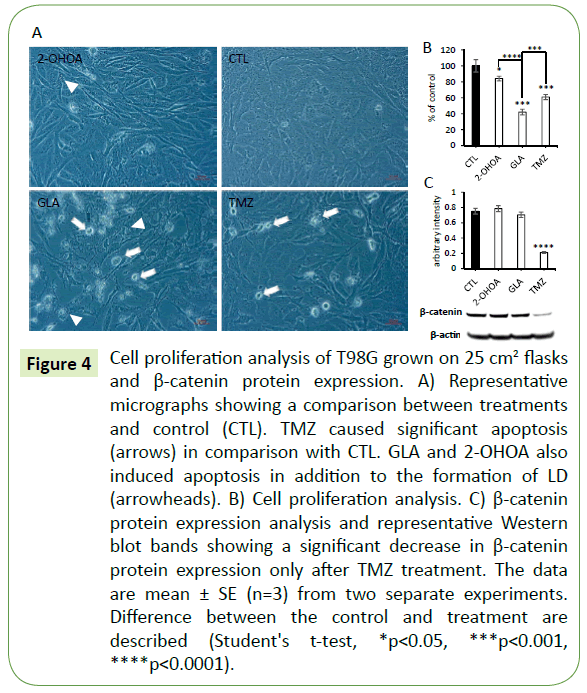
MTT cell viability and trypan blue exclusion assays were performed to obtain the correspondent minimally effective concentrations of TMZ and FA able to inhibit T98G cell proliferation without significantly affecting Caco-2 or HUVEC cell monolayer (data not shown). The following concentrations were used in all the assays: 500 μM for TMZ, 150 μM for both 2-OHOA and GLA, and 2 mg/ml for FISH. The latter was not cytotoxic to T98G cells. It did not affect cell proliferation, cell viability or even cell morphology (data not shown). As expected, it also did not affect T98G cell proliferation in the gut or BBB models. However, FISH antagonized the effect of TMZ on T98G cell proliferation (see below). GLA, 2-OHOA and TMZ alone presented the same range of effect on these models - around 20% inhibition of T98G cell proliferation (Figures 2 and 3). When those treatments were added directly to the cells - without any cell barrier - GLA revealed to be the most effective in decreasing T98G cell proliferation (Figures 4A and 4B). In addition, it was possible to observe a great number of apoptotic cells (white arrows) only after GLA and TMZ treatments (Figure 4A).
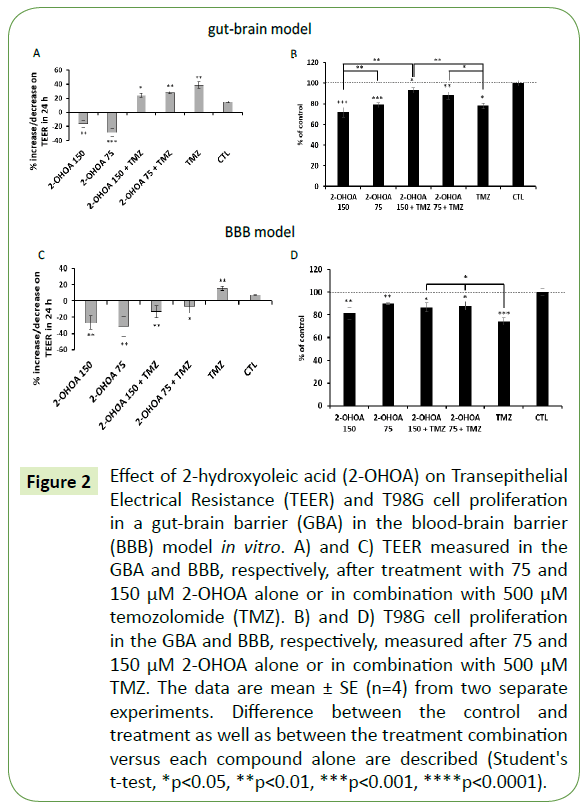
Figure 2: Effect of 2-hydroxyoleic acid (2-OHOA) on Transepithelial Electrical Resistance (TEER) and T98G cell proliferation in a gut-brain barrier (GBA) in the blood-brain barrier (BBB) model in vitro. A) and C) TEER measured in the GBA and BBB, respectively, after treatment with 75 and 150 μM 2-OHOA alone or in combination with 500 μM temozolomide (TMZ). B) and D) T98G cell proliferation in the GBA and BBB, respectively, measured after 75 and 150 μM 2-OHOA alone or in combination with 500 μM TMZ. The data are mean ± SE (n=4) from two separate experiments. Difference between the control and treatment as well as between the treatment combination versus each compound alone are described (Student's t-test, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001).
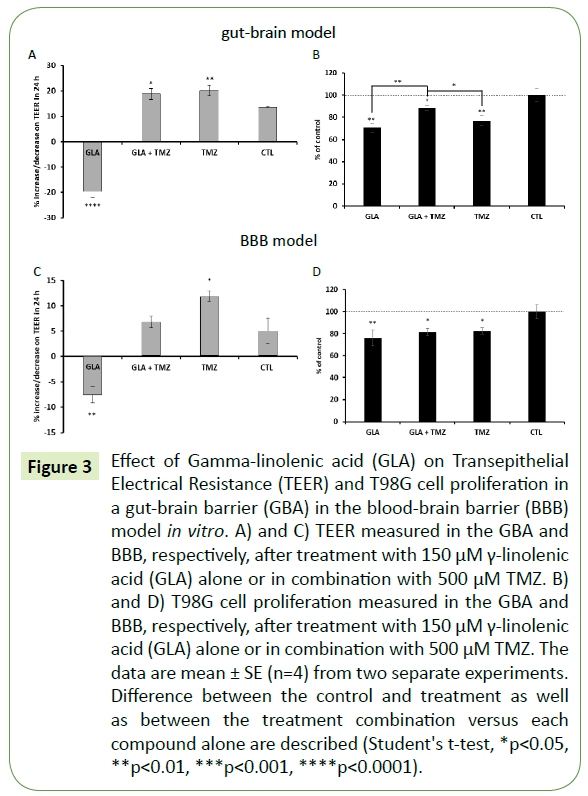
Figure 3: Effect of Gamma-linolenic acid (GLA) on Transepithelial Electrical Resistance (TEER) and T98G cell proliferation in a gut-brain barrier (GBA) in the blood-brain barrier (BBB) model in vitro. A) and C) TEER measured in the GBA and BBB, respectively, after treatment with 150 μM γ-linolenic acid (GLA) alone or in combination with 500 μM TMZ. B) and D) T98G cell proliferation measured in the GBA and BBB, respectively, after treatment with 150 μM γ-linolenic acid (GLA) alone or in combination with 500 μM TMZ. The data are mean ± SE (n=4) from two separate experiments. Difference between the control and treatment as well as between the treatment combination versus each compound alone are described (Student's t-test, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001).
Figure 4: Cell proliferation analysis of T98G grown on 25 cm2 flasks and β-catenin protein expression. A) Representative micrographs showing a comparison between treatments and control (CTL). TMZ caused significant apoptosis (arrows) in comparison with CTL. GLA and 2-OHOA also induced apoptosis in addition to the formation of LD (arrowheads). B) Cell proliferation analysis. C) β-catenin protein expression analysis and representative Western blot bands showing a significant decrease in β-catenin protein expression only after TMZ treatment. The data are mean ± SE (n=3) from two separate experiments. Difference between the control and treatment are described (Student's t-test, *p<0.05, ***p<0.001, ****p<0.0001).
2-OHOA effects in combination with TMZ
To test the effect of 2-OHOA on gut permeability we used a gut-brain axis in vitro model (Figure 1B). After 24-hour incubation, both 150 and 75 μM concentrations of 2-OHOA significantly increased Caco-2 cell-permeability, as demonstrated by TEER analysis (Figure 2A). A slight difference between 75 and 150 μM was observed (p=0.05), in which the lower concentration elicited a higher permeability of the Caco-2 cells. This effect was entirely reversed by combining 500 μM TMZ with 2-OHOA. The combination was able to increase TEER from -28.9% of the 75 μM 2-OHOA alone to +27.9% when combined. The combination also decreased T98G cell viability in the GBA model. As opposed to that, the combination a significantly reduced the inhibitory effect compared with TMZ or 2-OHOA alone (Figure 2B) thus exerting antagonistic effects when combined. We next examined the ability of 2-OHOA to cross the BBB using an in vitro BBB model (Figure 1B), in which a similar pattern was observed (Figure 2C) concerning TEER measurements. In this model, the combination of 2-OHOA and TMZ reduced the inhibitory effect of TMZ, but not that of 2-OHOA (Figure 2D).
FISH effects in combination with TMZ
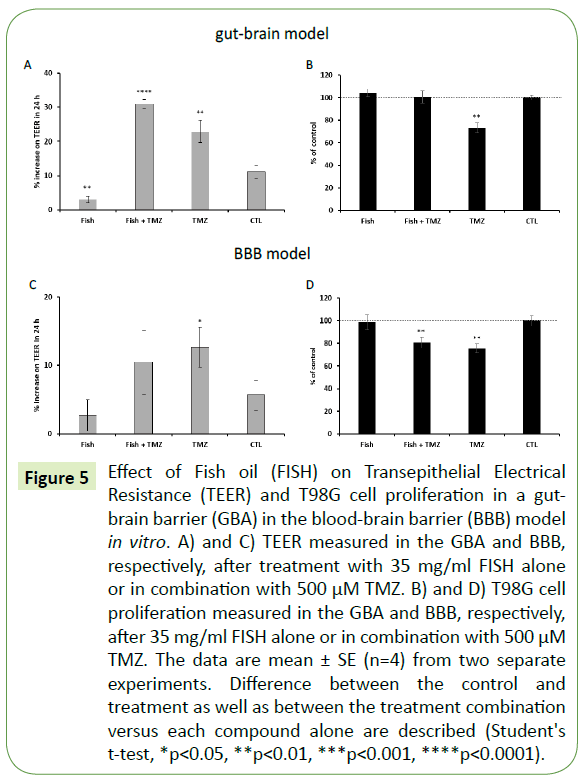
The same models were used to study the effects of omega-3 FA alone and in combination with TMZ. In the GBA model, FISH significantly halted the regular decrease of Caco-2 cell permeability in comparison with the control after 24 hours of incubation (Figure 5A). In the BBB model, FISH halted the general decrease of endothelial cell permeability after 24 hours of treatment as was previously observed in the GBA model (Figure 5A-C). TMZ alone significantly decreased endothelial cell permeability in the BBB model. Moreover, the combination of FISH with TMZ completely abolished the inhibitory effect of TMZ on T98G cell proliferation in the GBA model (Figure 5B). FISH alone was not effective in inhibiting T98G cell proliferation in comparison with TMZ alone. In the BBB model, FISH did not significantly interfere with the cytotoxic effect of TMZ on T98G cell proliferation (Figure 5D).
Figure 5: Effect of Fish oil (FISH) on Transepithelial Electrical Resistance (TEER) and T98G cell proliferation in a gut-brain barrier (GBA) in the blood-brain barrier (BBB) model in vitro. A) and C) TEER measured in the GBA and BBB, respectively, after treatment with 35 mg/ml FISH alone or in combination with 500 μM TMZ. B) and D) T98G cell proliferation measured in the GBA and BBB, respectively, after 35 mg/ml FISH alone or in combination with 500 μM TMZ. The data are mean ± SE (n=4) from two separate experiments. Difference between the control and treatment as well as between the treatment combination versus each compound alone are described (Student's t-test, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001).
GLA effects in combination with TMZ
Next, we tested the effects of the omega-6 FA, GLA. GLA significantly increased Caco-2 cell permeability similar to 2-OHOA, in the GBA model, while TMZ significantly decreased cell permeability (Figure 3A). In this model, the combination of TMZ with GLA showed no different effect on Caco-2 cell permeability, since the same range was observed with TMZ alone. In the BBB model (Figure 3C) GLA increased endothelial cell permeability, while TMZ decreased it, just as in the GBA model. The combination of GLA and TMZ in the BBB model resulted in no significant alteration of endothelial cell permeability in comparison with control. Regarding T98G cell proliferation, the combination of GLA with TMZ in the GBA model was significantly less effective than each substance alone (Figure 3B). Moreover, in the BBB model (Figure 3D), the combination of GLA with TMZ did not add to the antiproliferative effect of each substance alone. This means that all the inhibitory effects caused by each substance compared with the control were in the same range of the viable cells (GLA: 75.8%, GLA + TMZ: 81.2% and TMZ: 82.2%), with no significant differences between them.
Effect of TMZ, 2-OHOA and GLA on β-catenin and E-cadherin protein expression
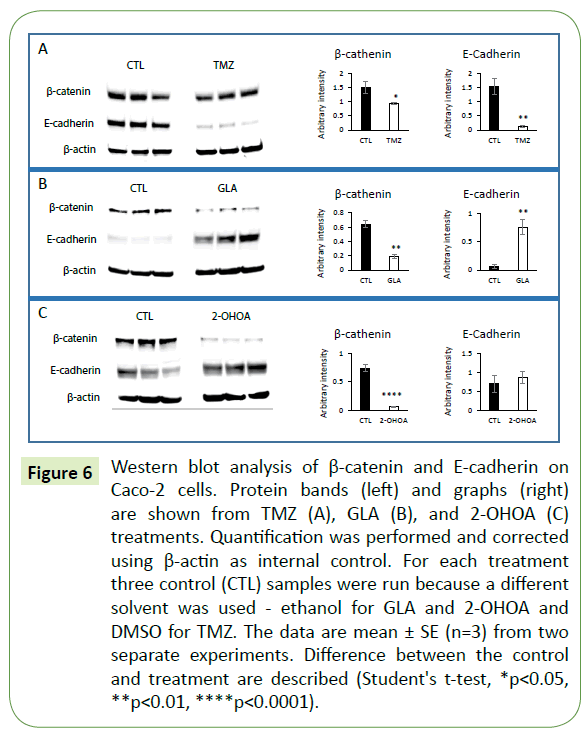
In order to explore the mechanism of action of TMZ, GLA and 2-OHOA we tested the expression pattern of the specific adherens junction (AJ)-related proteins, β-catenin and E-cadherin, in Caco-2 cell monolayers. Western blot analysis was performed using Caco-2 cell extracts previously treated with TMZ, GLA or 2-OHOA in triplicate, and was compared to control. Results showed that GLA, 2-OHOA and TMZ, were able to significantly decrease β-catenin protein expression (Figure 6A). Moreover, GLA significantly upregulated E-cadherin protein expression (Figure 6B), while TMZ downregulated it (Figure 6A). 2-OHOA did not significantly change E-cadherin protein expression (Figure 6C). In T98G cells (Figure 4C), only TMZ significantly decreased β-catenin protein expression - around 75% decrease - in relation to control. T98G cells do not express E-cadherin.
Figure 6: Western blot analysis of β-catenin and E-cadherin on Caco-2 cells. Protein bands (left) and graphs (right) are shown from TMZ (A), GLA (B), and 2-OHOA (C) treatments. Quantification was performed and corrected using β-actin as internal control. For each treatment three control (CTL) samples were run because a different solvent was used - ethanol for GLA and 2-OHOA and DMSO for TMZ. The data are mean ± SE (n=3) from two separate experiments. Difference between the control and treatment are described (Student's t-test, *p<0.05, **p<0.01, ****p<0.0001).
Effect of TMZ, 2-OHOA and GLA on cell morphology and induction of lipid droplets formation
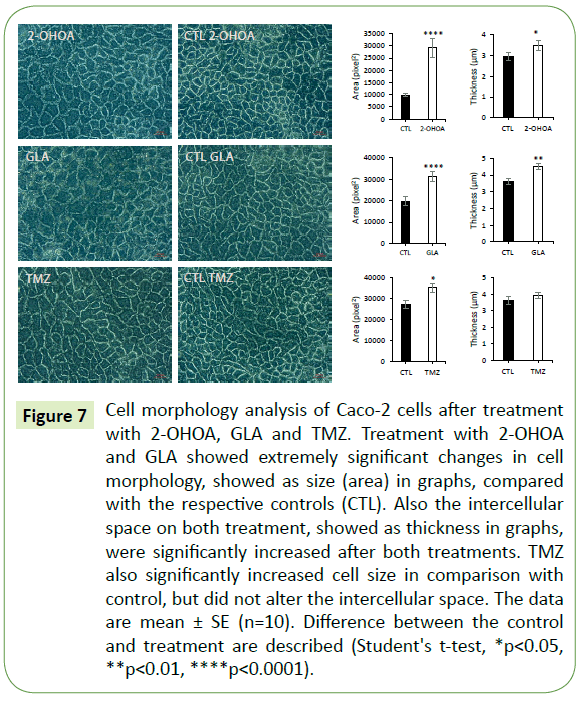
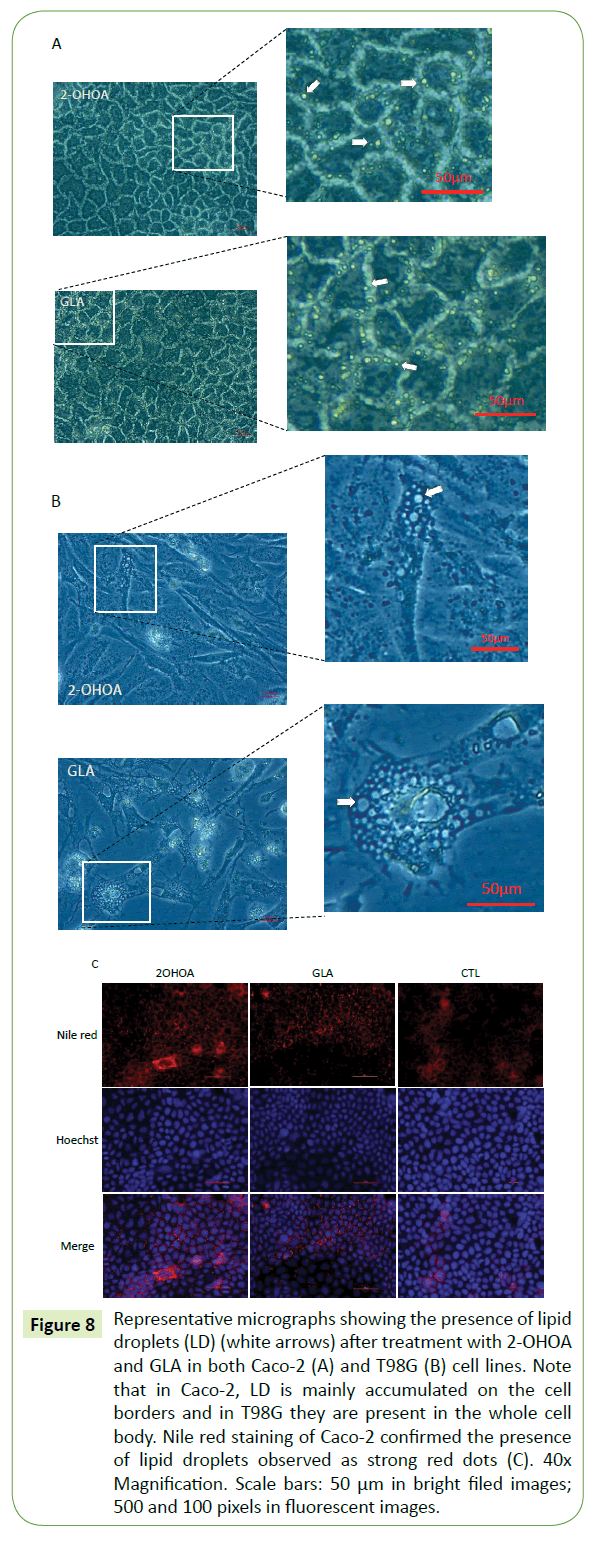
All three molecules caused significant increase in cell size in comparison to the respective controls. However, only GLA and 2-OHOA induced a significant increase in intercellular space (Figure 7). Additionally, it was observed in the brightfield lighting method, the formation of lipid droplets which in Caco-2 were abundant in cell borders (Figure 8A), while in T98G, it appeared in the whole cell body (Figure 8B). Nile red staining confirmed the presence of lipid droplets in Caco-2 cells (Figure 8C).
Figure 7: Cell morphology analysis of Caco-2 cells after treatment with 2-OHOA, GLA and TMZ. Treatment with 2-OHOA and GLA showed extremely significant changes in cell morphology, showed as size (area) in graphs, compared with the respective controls (CTL). Also the intercellular space on both treatment, showed as thickness in graphs, were significantly increased after both treatments. TMZ also significantly increased cell size in comparison with control, but did not alter the intercellular space. The data are mean ± SE (n=10). Difference between the control and treatment are described (Student's t-test, *p<0.05, **p<0.01, ****p<0.0001).
Figure 8: Representative micrographs showing the presence of lipid droplets (LD) (white arrows) after treatment with 2-OHOA and GLA in both Caco-2 (A) and T98G (B) cell lines. Note that in Caco-2, LD is mainly accumulated on the cell borders and in T98G they are present in the whole cell body. Nile red staining of Caco-2 confirmed the presence of lipid droplets observed as strong red dots (C). 40x Magnification. Scale bars: 50 μm in bright filed images; 500 and 100 pixels in fluorescent images.
Discussion
There is worldwide concern regarding over-the-counter use of FA as a dietary supplement. Pharmacological interactions with medications may occur, leading to potential dangerous and even life-threatening consequences [34]. Dietary supplements may have an effect on the pharmacokinetics of concomitant medications [34]. Due to ubiquitous marketing and increased exposure via the internet, consumers may believe that a “natural” product, such as herbal supplements or fish oil, is harmless. The global herbal supplement market will reach 86.74 billion dollars (US) by 2022, according to Zion Market Research™. However, there is a paucity of safety and efficacy data as well as insufficient information on drug-drug and food-drug interaction. Here, we demonstrate for the first time that the combination of TMZ with both naturally occurring and synthetic FA molecules is by no means synergistic. There is a consistent antagonism between these compounds. All FAs used had an inhibitory effect on the cytotoxic function of TMZ in the tested models.
In both GBA and BBB models, 2-OHOA decreased cell permeability dose dependently. However, especially in the GBA model, TMZ effect on TEER completely reversed the effect of 2-OHOA, suggesting competition between the substances. In the BBB model, the combination of TMZ + 2-OHOA reduced only the inhibitory effect of TMZ, but not that of 2-OHOA alone. This result could be in part associated with the difference between BBB and GBA permeability, showing less permeability of the BBB model in vitro to 2-OHOA. Interestingly, in vivo studies with this compound have demonstrated its ability to penetrate the BBB by alterations in membrane properties [18]. Moreover, we showed here an evident antagonism between 2-OHOA and TMZ on the inhibitory effect of T98G cell viability in both GBA and BBB models in vitro. Curiously, in the GBA model, 2-OHOA not only inhibited the anti-proliferative effect of TMZ, but also TMZ antagonized the 2-OHOA anti-proliferative impact on T98G cells. If this antagonistic effect indeed translates to in vivo, it will undoubtedly exhibit negatively in the currently running phase IIB clinical trial mentioned in the introduction.
T98G was found resistant to FISH, which are sources of DHA and EPA. In our experiments, the effective concentration obtained for FISH were >100 μg/mL indicating no cytotoxic effect based on the criteria set by the National Cancer Institute, which states that only natural substances with EC50 <20 μg/mL are considered cytotoxic [35]. Furthermore, in agreement with our data, studies have demonstrated that using EPA in combination with irradiation resulted in a substantial radiosensitization for colon adenocarcinoma HT-29 and GBM U251 cells, but not for T98G cells [36]. Mulpur et al. observed that omega-3 supplements used by brain cancer patients did not improve their survival rate [25]. Taking these observations into account, we learned that T98G cells, as well as human brain cancers in general, are non-sensitive to fish-derived omega-3 FA. Even though omega-3 FA has failed to demonstrate any effect on T98G cell proliferation, we found that FISH exhibited the same result on TEER in both models, arresting the expected increase in comparison with control. This result is in agreement with previous studies showing that DHA and EPA have the ability to increase Caco-2 cell permeability [37]. However, the combination of TMZ and FISH, in both models, just confirmed the powerful ability of TMZ on increasing TEER.
GLA had similar effects as 2-OHOA. Both reduced TEER in the GBA model as well as in the BBB model, and both had a significant effect on the viability of T98G cells. However, combining GLA with TMZ caused a significantly less antiproliferative effect in comparison with each molecule alone in the GBA model but not in the BBB model. Noteworthy, 150 μM GLA caused the same effect as 500 μM TMZ on T98G cell proliferation in both in vitro barrier models. In in vivo conditions, the presence of a more consistent number of specific lipoprotein receptors at the BBB [38], which may hinder GLA entrance to the brain and consequently to the tumor, should be considered. In fact, this issue has contributed to hampering the oral use of GLA for gliomas [28]. So, improving GLA delivery to the CNS is a challenge to pursue. Once this problem is solved, it could work just as TMZ, with the asset of not being toxic to normal tissues [33,39]. Concerning the barrier permeability, GLA and TMZ showed opposite effects on TEER in both models. While GLA loosened tight junctions as observed by the significant decrease on TEER, TMZ strengthened it by increasing TEER. In agreement with our study, Usami et al. also demonstrated the effect of GLA in increasing Caco-2 cell permeability, they also observed that this effect is regulated by protein kinase C (PKC) [37]. A study conducted by Yamagata and colleagues, demonstrated that GLA at 10 μM caused an increase on TEER in an in vitro barrier model using porcine brain capillary endothelial cells [40]. This is not in agreement with our results. However, results may differ depending on cell lines and the concentration of GLA used. Additionally lower concentrations like the one used by Yamagata and colleagues are known to be non-cytotoxic to glioma cells in vitro or in vivo [29,30]. The concentrations used in our model are more relevant to clinical translation. Moreover, TMZ in combination with GLA, in the GBA model, was still able to reduce Caco-2 cell permeability at the same range as TMZ alone, suggesting a predominant effect by TMZ on this barrier. However, in the BBB model, GLA apparently interfered with the effects of TMZ on endothelial cell permeability. These results are in line with existing data showing the ability of GLA to inhibit the impact of some chemotherapies. It has been reported that the association between Fotemustine and GLA salts have antagonistic effects [41].
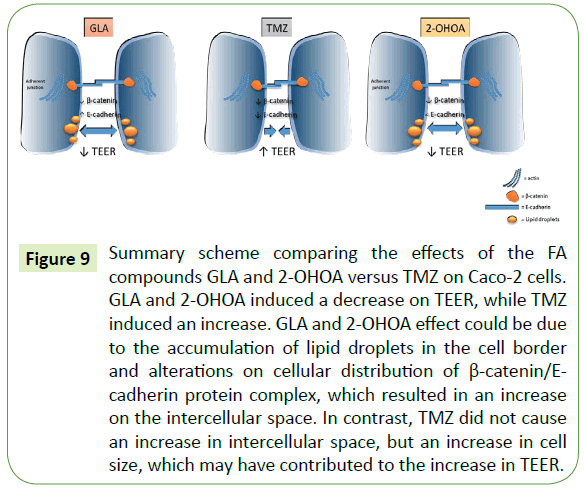
A striking finding of this study happened as we continued to explore and compare the mechanism of action of TMZ, GLA and 2-OHOA, which induced a significant and consistent effect in comparison to FISH. We started investigating the expression pattern of the specific adherens junction (AJ)-related proteins, β-catenin and E-cadherin, in Caco-2 cell monolayers. The membrane phospholipid composition plays an essential role in the in vivo maintenance of AJ structures involved in cell– cell adhesion [42]. Thus, we inferred that incubation with GLA or 2-OHOA could induce alterations in AJ-related protein expression leading to redistribution of β-catenin/E-cadherin protein complex in cell membranes. Indeed, we observed that GLA and 2-OHOA significantly decreased β-catenin protein expression, and the expression of E-cadherin was significantly upregulated by GLA but not by 2-OHOA (Figure 6). TMZ per se also significantly decreased β-catenin, but different from GLA and 2-OHOA, it significantly downregulated E-cadherin protein expression. Interestingly, our morphology studies revealed that the size (area) of cells was significantly increased by all three compounds tested, while the intercellular space was only augmented by GLA and 2-OHOA (Figure 7). Also, a massive accumulation of lipid droplets (LD) was observed on Caco-2 cell borders after incubation with GLA and 2-OHOA. Nile red staining was used to confirm the presence of LD on Caco-2 cells (Figure 8C). The same effect was also observed in T98G cells, with the difference that the LD were found in the whole cell body (Figure 8B). Taken together, the increase on intercellular space and the accumulation of LD at the cell junctions of Caco-2 may explain at least in part the observed decrease on TEER after treatment with both 2-OHOA and GLA molecules (Figure 9). Incubation with TMZ led to a significant increase in cell size (Figure 7) and showed no significant changes in the intercellular space in comparison with control. It could be inferred that the increase in cell size by itself has contributed to the observed increase on TEER, when taking into account the whole space of the well. Additional protein expression analysis revealed that TMZ, but not GLA and 2-OHOA, induced a significant inhibition of β-catenin in T98G cells (Figure 4C). The overall findings corroborate the fact that TMZ has a distinct mechanism of action and also antagonistic effects in relation to GLA or 2-OHOA. Additionally, based on the effect of GLA on T98G cell proliferation (Figure 4B), we may consider GLA as the best candidate for treating glioma cells, pending on the development of delivery systems in order to effectively increase its effect in the brain.
Figure 9: Summary scheme comparing the effects of the FA compounds GLA and 2-OHOA versus TMZ on Caco-2 cells. GLA and 2-OHOA induced a decrease on TEER, while TMZ induced an increase. GLA and 2-OHOA effect could be due to the accumulation of lipid droplets in the cell border and alterations on cellular distribution of β-catenin/E-cadherin protein complex, which resulted in an increase on the intercellular space. In contrast, TMZ did not cause an increase in intercellular space, but an increase in cell size, which may have contributed to the increase in TEER.
In general, FA can be incorporated in cell membrane, causing alterations in membrane composition and fluidity, as well as changes to substrate-receptor interactions [19,20,43]. This may lead to the antagonistic effects detected in this study. Future in vivo studies should clarify in more details the precise mechanism by which those FA molecules interfere with the mechanism of action of TMZ and vice-versa.
Conclusion
Using our in vitro barrier models, we conclude that FAs might negatively interfere with the cytotoxic effect of TMZ. On the other hand, TMZ may also play a negative role in the antitumor action of specific FAs like GLA or 2-OHOA. The current results raise questions concerning the clinical utilization of these FAs as nutritional adjuvants for patients under treatment with TMZ. It is plausible that patients with GBM should at least be treated with FA and TMZ separately. Likewise, other CA may present pharmacologic interactions with FA, indicating a need for additional studies in this field.
Acknowledgment
We thank Professor Alex Rouvinski from the Department of Microbiology and Molecular Genetics, Institute for Medical Research Israel-Canada, The Hebrew University of Jerusalem for the fluorescent microscope utilization.
References
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, et al. (2016) The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131: 803-820.
- Jovčevska I, Kočevar N, Komel R (2013) Glioma and glioblastoma - how much do we (not) know? Mol Clin Oncol 1: 935-941.
- Yan Y, Xu Z, Li Z, Sun L, Gong Z (2017) An insight into the increasing role of LncRNAs in the pathogenesis of gliomas. Front Mol Neurosci10: 53.
- Clarke JL, Ennis MM, Yung WKA, Chang SM, Wen PY, et al. (2011) Is surgery at progression a prognostic marker for improved 6-month progression-free survival or overall survival for patients with recurrent glioblastoma? Neuro Oncol 13: 1118-1124.
- Mrugala MM (2013) Advances and challenges in the treatment of glioblastoma: a clinician’s perspective. Discov Med 15: 221-230.
- Iacob G, Dinca EB (2009) Current data and strategy in glioblastoma multiforme. J Med Life 2: 386-393.
- Mallick S, Benson R, Hakim A, Rath GK (2016) Management of glioblastoma after recurrence: a changing paradigm. J Egypt Natl Canc Inst 28: 199-210.
- Stark AM, van de Bergh J, Hedderich J, Mehdorn HM, Nabavi A (2012) Glioblastoma: clinical characteristics, prognostic factors and survival in 492 patients. Clin Neurol Neurosurg 114: 840-845.
- Rahman R, Catalano PJ, Reardon DA, Norden AD, Wen PY, et al. (2015) Incidence, risk factors, and reasons for hospitalization among glioblastoma patients receiving chemoradiation. J Neurooncol 124: 137-146.
- Parasramka S, Talari G, Rosenfeld M, Guo J, Villano JL (2017) Procarbazine, lomustine and vincristine for recurrent high-grade glioma. Cochrane Database Syst Rev 7: CD011773.
- Hanif F, Muzaffar K, Perveen K, Malhi SM, Simjee SU (2017) Glioblastoma multiforme: a review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac J Cancer Prev 18: 3-9.
- Yan Y, Xu Z, Dai S, Qian L, Sun L, et al. (2016) Targeting autophagy to sensitive glioma to temozolomide treatment. J Exp Clin Cancer Res 35: 23.
- Dai S, Yan Y, Xu Z, Zeng S, Qian L, et al. (2017) SCD1 Confers temozolomide resistance to human glioma cells via the Akt/GSK3β/β-catenin signaling axis. Front Pharmacol 8: 960.
- Sengupta S, Marrinan J, Frishman C, Sampath P (2012) Impact of temozolomide on immune response during malignant glioma chemotherapy. Clin Dev Immunol 2012: 1-7.
- Dario A, Tomei G (2006) The safety of the temozolomide in patients with malignant glioma. Curr Drug Saf 1: 205-222.
- Singhal N, Selva-Nayagam S, Brown MP (2007) Prolonged and severe myelosuppression in two patients after low-dose temozolomide treatment- case study and review of literature. J Neurooncol 85: 229-230.
- https://www.openaire.eu/search/project?projectId=corda__h2020::e643da74943fa7bff94244aa422ff497
- Torgersen ML, Klokk TI, Kavaliauskiene S, Klose C, Simons K, et al. (2016) The anti-tumor drug 2-hydroxyoleic acid (Minerval) stimulates signaling and retrograde transport. Oncotarget 7: 86871-86888.
- Escribá PV, Busquets X, Inokuchi J, Balogh G, Török Z, et al. (2015) Membrane lipid therapy: modulation of the cell membrane composition and structure as a molecular base for drug discovery and new disease treatment. Prog Lipid Res 59: 38-53.
- Lladó V, López DJ, Ibarguren M, Alonso M, Soriano JB, et al. (2014) Regulation of the cancer cell membrane lipid composition by NaCH Oleate. Biochim Biophys Acta - Biomembr 1838: 1619-1627.
- Fan H-C, Chi C-S, Chang Y-K, Tung M-C, Lin S-Z, et al. (2018) The molecular mechanisms of plant-derived compounds targeting brain cancer. Int J Mol Sci 19: 395.
- Park MN, Song HS, Kim M, Lee M-J, Cho W, et al. (2017) Review of natural product-derived compounds as potent antiglioblastoma drugs. Biomed ResInt 2017: 1-24.
- Zhang Y, Wang S-X, Ma J-W, Li H-Y, Ye J-C, et al. (2015) EGCG inhibits properties of glioma stem-like cells and synergizes with temozolomide through downregulation of P-glycoprotein inhibition. J Neurooncol 121: 41-52.
- Nabavi SF, Bilotto S, Russo GL, Orhan IE, Habtemariam S, et al. (2015) Omega-3 polyunsaturated fatty acids and cancer: lessons learned from clinical trials. Cancer Metastasis Rev 34: 359-380.
- Mulpur BH, Nabors LB, Thompson RC, Olson JJ, LaRocca RV, et al. (2015) Complementary therapy and survival in glioblastoma. Neuro-Oncol Pract 2: 122-126.
- Bartsch H, Nair J, Owen RW (1999) Dietary polyunsaturated fatty acids and cancers of the breast and colorectum: emerging evidence for their role as risk modifiers. Carcinogenesis 20: 2209-2218.
- Kyritsis A, Bondy M, Levin V (2011) Modulation of glioma risk and progression by dietary nutrients and antiinflammatory agents. Nutr Cancer 63: 174-184.
- Das UN (2007) Gamma-linolenic acid therapy of human glioma-a review of in vitro, in vivo and clinical studies. Med Sci Monit 13: RA119-131.
- Benadiba M, Miyake JA, Colquhoun A (2009) Gamma-linolenic acid alters Ku80, E2F1, and bax expression and induces micronucleus formation in C6 glioma cells in vitro. IUBMB Life 61: 244-251.
- Miyake JA, Benadiba M, Colquhoun A (2009) Gamma-linolenic acid inhibits both tumour cell cycle progression and angiogenesis in the orthotopic C6 glioma model through changes in VEGF, Flt1, ERK1/2, MMP2, cyclin D1, pRb, p53 and p27 protein expression. Lipids Health Dis 8: 8.
- Preuss M, Girnun GD, Darby CJ, Khoo N, Spector AA, et al. (2000) Role of antioxidant enzyme expression in the selective cytotoxic response of glioma cells to gamma-linolenic acid supplementation. Free Radic Biol Med 28: 1143-1156.
- Vartak S, Robbins ME, Spector AA (1999) The selective cytotoxicity of gamma-linolenic acid (GLA) is associated with increased oxidative stress. Adv Exp Med Biol 469: 493-498.
- Vartak S, McCaw R, Davis CS, Robbins ME, Spector AA (1998) Gamma-linolenic acid (GLA) is cytotoxic to 36B10 malignant rat astrocytoma cells but not to “normal” rat astrocytes. Br J Cancer 77: 1612-1620.
- Mouly S, Lloret-Linares C, Sellier P-O, Sene D, Bergmann J-F (2017) Is the clinical relevance of drug-food and drug-herb interactions limited to grapefruit juice and Saint-John’s Wort? Pharmacol Res 118: 82-92.
- Puškárová A, Bučková M, Kraková L, Pangallo D, Kozics K (2017) The antibacterial and antifungal activity of six essential oils and their cyto/genotoxicity to human HEL 12469 cells. Sci Rep 7: 8211.
- Manda K, Kriesen S, Hildebrandt G, Fietkau R, Klautke G (2011) Omega-3 fatty acid supplementation in cancer therapy. Strahlentherapie und Onkol 187: 127-134.
- Usami M, Komurasaki T, Hanada A, Kinoshita K, Ohata A (2003) Effect of gamma-linolenic acid or docosahexaenoic acid on tight junction permeability in intestinal monolayer cells and their mechanism by protein kinase C activation and/or eicosanoid formation. Nutrition 19: 150-156.
- Edmond J (2001) Essential polyunsaturated fatty acids and the barrier to the brain: the components of a model for transport. J Mol Neurosci 16: 181-194.
- Bakshi A, Mukherjee D, Bakshi A, Banerji AK, Das UN (2003) Gamma-linolenic acid therapy of human gliomas. Nutrition 19: 305-309.
- Yamagata K, Tagami M, Takenaga F, Yamori Y, Nara Y, et al. (2003) Polyunsaturated fatty acids induce tight junctions to form in brain capillary endothelial cells. Neuroscience 116: 649-656.
- Ilc K, Ferrero JM, Fischel JL, Formento P, Bryce R, et al. (1999) Cytotoxic effects of two gamma linoleic salts (lithium gammalinolenate or meglumine gammalinolenate) alone or associated with a nitrosourea: an experimental study on human glioblastoma cell lines. Anticancer Drugs 10: 413-417.
- Márquez MG, Favale NO, Leocata Nieto F, Pescio LG, Sterin-Speziale N (2012) Changes in membrane lipid composition cause alterations in epithelial cell–cell adhesion structures in renal papillary collecting duct cells. Biochim Biophys Acta - Biomembr 1818: 491-501.
- Colquhoun A, Miyake JA, Benadiba M (2009) Fatty acids, eicosanoids and cancer. Nutr Ther Metab 27: 105-112.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences