Stem Cells Therapy: A Promising Cure for Parkinson's Disease or Not? A Review
Ravikant* and Sneha GuptA
Department of Science Education and Research, National Institute of Pharmaceutical Education and Research, Hajipur, India
Published Date: 2023-02-28DOI10.36648/2573-5349.8.2.001
Ravikant* and Sneha Gupta
Department of Science Education and Research, National Institute of Pharmaceutical Education and Research, Hajipur, India
- *Corresponding Author:
- Ravikant
Department of Science Education and Research,
National Institute of Pharmaceutical Education and Research,
Hajipur,
India,
Tel: 8059533063;
Email: rkravikant910@gmail.com
Received: July 29, 2021, Manuscript No. IPNBT-23-16106; Editor assigned: August 03, 2021, PreQC No. IPNBT-23-16106 (PQ); Reviewed: August 17, 2021, QC No. IPNBT-23-16106; Revised: January 31, 2023, Manuscript No. IPNBT-23-16106 (R); Published: February 28, 2023, DOI: 10.36648/2573-5349.8.2.001
Citation: Ravikant, Gupta S (2023) Stem Cells Therapy: A Promising Cure for Parkinson’s Disease or not? A review. J Transl Neurosc Vol. 8 No. 2: 001.
Abstract
Parkinson's disease is the most common movement disorder and most common degenerative illness of the CNS. Parkinson's Disease (PD) is the most common movement disorder and the second most common degenerative illness of the central nervous system. A decrease in the concentration of dopaminergic neurons in the pars compacta region decreases the ability to move the body. Another major key risk for PD progression is age. The first PD treatment began in 1960 when it was found that patients lacked neuronal dopamine. Mitotic cell division enables cells to divide and differentiate into a wide range of specialized cell types. Parkin trafficking to mitochondria is impaired by PINK1 mutations. Following transplantation of human iPSC-derived NPCs in a monkey model, DA neurons have been demonstrated to develop. Like other adult stem cells, MSCs are capable of self-renewal despite being multipotent and capable of differentiating into osteocytes and astrocytes. BMSCs might be utilized to produce dopamine neurons selectively. Induction is achieved by lipofection of a plasmid expressing a Notch1 Intracellular Domain (NICD) and G418 screening, followed by feeding a specific combination of trophic chemicals and cytokines. NSCs were described as granule cells with a high proportion of cell proliferation in the cerebral cortex, subventricular zone and hippocampal area that may readily develop into astrocytes, oligodendrocytes and neurons.
Keywords
Parkinson’s; Stem cells; Neurogenesis; Cell replacement therapy
Abbreviations
PD: Parkinson’s disease; hESC: Human Embryonic Stem Cells; hiPSCs: Human Induced Pluripotent Stem Cells; MSCs: Mesenchymal Stem Cells; bMSCs: Bone Marrow Stem Cells; SNCA-α-synuclein; GBA: Glucocerebrosidase; LRRK2: Leucine Rich Repeat Kinase 2; VPS35: Vacuolar Protein Sorting Associated Protein 35; PARK2: Parkin RBR E3 ubiquitin protein ligase
Introduction
Parkinson's disease is the most prevalent motion disorder and the second most prevalent central nervous system degenerative condition. This condition was first defined in 1817 by James Parkinson in his essay on the shaking palsy, meticulously detailing the key motor symptoms of the disease that are now regarded as the hallmarks of PD are bradykinesia, stiffness and tremor. Furthermore, mental signs of the illness were observed. The development of α-synuclein which contains lewy bodies in the substantia nigra part of the brain characterizes PD neuropathologically. A depletion in the concentration of dopaminergic neurons in the pars compacta area reduces the enablement of body movement. The deposition of α synuclein in the brain increases as PD progresses and non motor symptoms in PD have attracted a lot of attention in the last decade [1]. Nonetheless, the motor symptoms of this disease, which cause PD to be a mobility disorder, continue to be the disease's key components and the most important characteristics for a diagnosis of PD even with modern imaging or laboratory tests to aid in the diagnostic challenge. For parent child PD, seven causal genes have been identified along with α Synuclein (SNCA), Glucocerebrosidase (GBA), Leucine Rich Repeat Kinase 2 (LRRK2), Vacuolar Protein Sorting associated protein 35 (VPS35), Parkin RBR E3 ubiquitin-protein ligase (PARK2), phosphatase and tensing homolog-induced kinase acts as a biomarker in the progression of PD. Early detection is essential because approximately 70% of neuronal damage occurs before the start of symptoms [2]. Factors such as age, gender and nationality may all influence PD risk. Age is a significant risk factor for PD. Current treatments for PD comprise symptomatic therapy with a combination of L-DOPA and carbidopa, which enhance dopamine synthesis and release and are especially successful in lowering akinesia and stiffness throughout the early phases of the illness. Embryonic stem cells are type of pluripotent cells which were extracted from the blastocyts inner mass of cells from which all cells in the body are derived. Such cells are distinct from zygotes that they are unable to produce an additional embryonic tissue [3].
Literature Review
Epidemiology
Breakthroughs have transformed our understanding of PD and its drivers. Although gene studies showed the variability of PD and offered clues into its pathophysiology and symptomatology, epidemiological studies have given strong evidence that psychosocial and environmental components have a major role in the etiology and development of the disease [4]. This data is reinforced and supplemented by the fact that 90 percent of patients have no apparent hereditary cause and that multiple factors contribute to the condition linked to an increased risk of PD in animals, exhibit neuroprotective or neurotoxic characteristics disease models. PD is the most prevalent degenerative illness at 2nd position (after Alzheimer's), with a typical years-old yearly prevalence rate in large nations of 14 per lakh persons in the general population and 160 per lakh populations aged 65 or more than it. A potentially higher comprehensible metric of illness prevalence is throughout the lifetime, which has been predicted to be 2 percent of total males and 13 percent of total women in the United States for persons aged forty years, after accounting for opposing risk for eg., additional causes of mortality, such as cardiovascular disease or cancer [5]. In Africa, age adjusted PD occurrence is low which includes deaths and prevalence in comparison to Europe and the USA but in the case of Asia, the scenario is the same. In a survey of US Medicare beneficiaries, the prevalence was also greater in white individuals than that in black or Asian individuals. From 1999 to 2009, the UK had a 6% annual decrease in the prevalence of PD which was linked to improved identification of various pathologies, since the total prevalence of PD remained steady. PD is unusual before even the age of 50, but it rises fast with ages, culminating throughout most research from around the age of 80, owing to undiagnosed [6].
Discussion
Cell replacement therapy
The first therapy for PD began in 1960 when it was discovered that afflicted people lacked neuronal dopamine. Physicians gave injectable L-Dopa and symptoms improved almost immediately. For many years, L-Dopa therapy was the benchmark for the treatment of PD, but now several commercially accessible medicines address PD without a dopaminergic mode of action [7]. This placed a strong emphasis on the DA system also aided in revealing the movement network of the basal ganglia by boosting investment to comprehend the wider interaction of PD pathogenesis. Deep brain stimulation through brain impulses to the inner section of the globus pallidus and subthalamic nucleus was presented as a supplementary intervention with drastically favourable outcomes with a new understanding of the afflicted circuits. Numerous studies, as with most neurological diseases, have proven the relative efficacy of non-medical/surgical treatments such as exercising, dancing and psychotherapy [8]. Cell replacement therapy has utilized foetal mesencephalic tissues to restore the dopamine producing neurons eliminated throughout the development of PD and to initiate innervation these cells inside the midbrain area have been grafted. The capacity of stem cells to regenerate themselves distinguishes them. Mitotic cell division enables cells to divide and differentiate into a wide range of different and special cell types. They are categorized based on their ability to develop into specialized cells. First type is totipotent stem cells, which may produce a fully functional creature, including placental cells [9]. The zygote and the cells in their very early stages after fertilization are thought to be totipotent. Pluripotent stem cells, on the other hand, can grow into specialized cells of such three primary germ layers (ectoderm, mesoderm, and endoderm), except for extraembryonic organs such as the placenta. To date, best cells are Embryonic Stem Cells (ESCs), which are generated from the blastocyst's inner cell mass. In principle, because of their properties, these cells might be an ideal source of DA neurons for stem cells and nevertheless, to achieve this aim, an effective approach for differentiating to operational striatum DA neurons is necessary [10]. Indeed, a variety of techniques have been used to produce cultures abundant in adult DA neurons from ESC, including stromal cells co culture, growth factors, secretory factors, transcription factors, and morphogens, with several positive effects indicated after implantation of such cells in animal studies of PD. New developments in stem cell research have resulted in tools for reprogramming normal adult somatic cells to pluripotency [11]. The very first described lineages of reprogrammed cells are known as induced pluripotent stem cells, were created by injecting 4 transcriptional factors inside mature fibroblasts such as Oct3/4, Sox2 and Klf4. In terms of shape, gene expression profile and differentiation capacity, these cells are comparable to hESC. Because these cells might be utilized as an in vitro cellular model of PD and for autologous transplantation, induced iPSC technology opens new avenues for biological research and therapeutic applications [12]. As a result, obtaining an effective and rigorous differentiation procedure of hiPSC into midbrain DA-like neurons is critical. Furthermore, because hiPSC are generated from somatic cells using standard tissue donation techniques, they do not create ethical issues. The next kind of stem cell is multipotent stem cells, which exclusively create cell lineages, such as neural stem cells which have been generated through neural tissues. Such cells have the potential of self renewal and develop into neural progenitor cells, which can give birth to various types of cells such as neurons, astrocytes and oligodendrocytes [13]. The generation of disease neurological subtypes, namely DA neurons, by differentiated iPSCs in vitro is a critical first step toward PD modelling. In vitro, prenatal introduction to noggin antagonists of BMP communication enables for very effective feeder free induction of neurons in adherent cells and enables for dopaminergic and motoneuronal potentials. On contrary, neuronal stimulation may be achieved in the lack of stimuli and by co culturing ES with stromal feeder cell lines. Following that, FGF8, FGF2, SHH, GNDF, BDNF along vitamin c work together to create DA patterning [14]. Ultimately, complete differentiation occurs following the removal of SHH and FGF8 which is aided by the presence of vitamin C, GDNF, TGFb-1, cyclic-AMP and Wnt5 (Figure 1).
iPSCs
Yamanaka's team revealed in 2006 that a combination of four transcription factors, namely OCT4, SOX2, KLF4 and c-Myc, would resynchronize epidermal fibroblast to pluripotent stem cells. Those cells showed potential to get developed into distinct types of cells from the three major germ layers, which are important characteristics of ESCs. For reprogramming, researchers have been experimenting with several cell sources [15]. iPSCs could now be created from a variety of sources, including hepatic, gastric, brain stem cells, and circulating blood cells. The effectiveness of peripheral blood cells as reprogramming models provokes therapeutic interest because collecting blood is the least intrusive procedure and hardly ever causes problems. iPSCs allow researchers to validate their understanding of PD derived from biological or experimental models, as well as pathological investigations in living DA neurons. For illustration, a most recent intriguing revelation on PD cause is the involvement of mitochondrial degradation. When the mitochondrial membrane depolarizes, the PINK1 aggregates on the outer membrane of mitochondria attracting parkin and starting mitochondrial degradation. Rodent DA neurons may be different from the human type of neurons [16]. As a result, it is critical to confirm mitochondrial degradation in human DA neurons. Mutations in PINK1 were shown to impair parkin trafficking to mitochondria, subsequently limiting apoptotic elimination of mitochondria in neurons derived from iPSC. In contrast to damage to the CNS where following glial growth finally entraps the lesion, transplanted neurons in PD may have a higher probability of correcting for decreased neurons without any of the obstacles of glial scarring. Furthermore, transplantation of hESCs has resulted in awe inspiring results in PD therapy. Furthermore, embryonic or foetal neural transplantation operations are cut short due to tissue accessibility, possible immune rejection and ethical issues [17]. However, because iPSCs have such an infinite proliferative potential, they can provide an endless supply of neurons for tissue regeneration. It also eliminates ethical issues, such as those with foetal tissues. Ground-breaking research demonstrated that transplanted iPSCs could develop into DA neurons and restore motor impairments in a rat PD model. Following that, DA neurons have been shown to develop from human iPSC-derived NPCs following transplant in a monkey model. Most DA neurons persisted for up to six months, although the enhancement was minimal. Protein based induced iPSCs after being exposed to DA neurons, NPCs have been shown to develop into DA neurons [18]. These are all the outcomes that give a positive prognosis for the use of transplanting application of iPSCs to PD therapy. A clinical trial strategy is recommended for iPSC-based cellular therapies. Astrocytes are the most numerous kind of cells in the central nervous system, serving a variety of tasks including functional and structural assistance for neurons. An increasing body of information shows that astrocyte malfunction contributes to the development of familial PD. DJ-1, SNCA, PLA2G6, LRRK2 and GBA mutations cause aberrant glutamate intake, mitochondrial dysfunction, inflammatory reaction, water transportation deficiency, and autophagy loss. Neuron maturation was shown to be significantly increased in a coculture system comprising astrocytes and neurons at a 60:40 ratio as per neurophysiological maturation. As a result, astrocytes are commonly utilized in cellular modeling methods to understanding neurological disorders. Likewise, glial cells may aid DA neurons in their protection against neurotoxins while also reducing the dysfunction of mitochondria. Surprisingly, the glial cells and neuron co culture method enhanced neuron marker proliferation while also stabilizing mitochondrial function via ROS reduction and boosted the activity of mitochondria. PD is also a possible contender for cell treatment because of its relatively localized neurodegeneration. In 1987, foetal ventral midbrain tissue was transplanted in PD patients and the study findings indicated cell proliferation and DA neuron functioning even after twenty years of transplantation in certain individuals.
Challenges of using iPSCs in treatment of Parkinson’s
Motor control, social behavior and memory are all modulated by DA neurons. Restatement of a single cell type maturation characteristics is a potent technique for influencing cell fate choice throughout the human iPSC differentiation process in vivo.
Including both research and therapeutic purposes, in vitro production of functioning DA neurons is important in the biology of a pluripotent cell. Two effective procedures for producing DA neurons are the Neural Stem Cell (NSPC) technique and the floor plate cell methodology. The NSPC method, which isolates NSPCs from rosettes, is frequently employed in neuronal differentiation. Hynes et al. introduced the floor plate approach, which is based on the notion that the floor plate is a key signaling hub during neuro development positioned with the ventral midline of the embryo. The midbrain DA neurons generated from the floorplate can control the release of dopamine and preferential reuptake of DA, as well as other characteristics such as signal transduction. Significantly, cellular PD symptoms such as the excessive proportion of ROS and intracellular α synuclein, mitochondrial DNA damage, shorter neurites and defective autophagy are observed in PD patient iPSC-derived DA neurons.
Disease phenotypes as a hurdle in cell therapy
The aging process is a significant risk factor for all delayed neurodegenerative diseases. One significant issue with iPSC- based PD models is accurately reproducing late onset symptoms. When somatic cells are reprogrammed into iPSCs, their individuality is reset to that of an embryo. The capacity of iPSCs to divide indefinitely while retaining genetic integrity offers a route around the aging barrier. Although these iPSCs grow into neuronal cells, it takes a long time to grow them to imitate aging DA neurons, posing a substantial challenge in simulating Parkinson's disease. Therefore, it is critical to understand how to promote aging in iPSC-derived DA neurons. Justine et al examined fibroblasts of adolescent and elder groups of the population and discovered a significant variation in progerin levels between the two groups. Researchers also showed that overexpression of progerin in PD iPSC-derived DA neurons in vitro or in vivo accelerated cell aging for mimicking late onset PD characteristics including severe dendritic deterioration, dramatic decrease of tyrosine hydroxylase activity and larger mitochondria or lewy body aggregates. Therefore, the expression of progerin may expedite aging in iPSC-derived DA neurons, producing pathological symptoms and adding progerin expression may be a viable method for manifesting disease phenotypes. Nevertheless, that neurons are too weak to carry out external gene transfer. The poor effectiveness of transfection in neurons remains a significant barrier to the broad and simple use of progerin expression to simulate late onset neurological disorders using iPSCs.
MScs
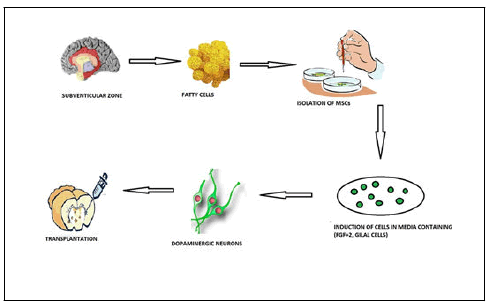
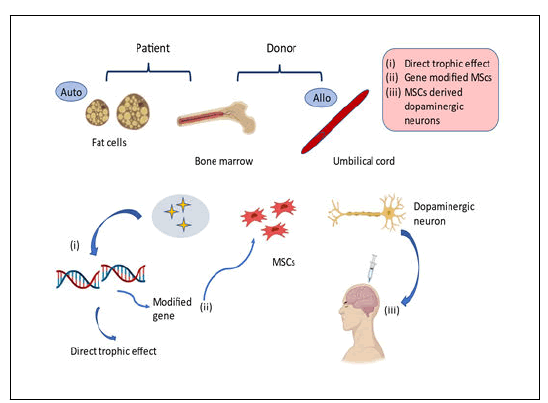
MSCs are the type of stem cells from the mesodermal lineage which commonly seen in the bone marrow and which can be isolated from the dermal layer, fatty tissues namely adipose, peripheral blood along the umbilical cord. MSCs feature long, narrow cells with such a massive nucleus, comparable to fibroblasts. MSCs, like other tissue stem cells, have a great ability for self renewal despite remaining multipotent and having the ability to get differentiate into osteocytes, astrocytes, etc. Unlike other stem cells, MSCs may be produced by both patients and normal donors. As a result, MSCs are a total viable source for regenerative medicine. MSCs have two primary impacts in cellbased therapy, a nutritive effect facilitated by the many kinds of factors and mediators generated by those cells and undergoes differentiation to form a wide range of cells for the replacement of depleted cells. Stem cells typically generate the sorts of cells found throughout the tissue wherein they reside, therefore their diversification possibilities are thought to be restricted. MSCs vary from conventional somatic stem cells in that, as previously noted, they develop not just to into the very same lineage of mesoderm, cartilage and adipose cells, as well additionally into lineages of endodermal and ectodermal cells too.
MSC flexibility and transdifferentiation possibilities were first characterized as a result of in vivo research in which differentiated donor bone marrow derived cells were implanted entering the donor brain's glial cells. While some studies have shown that these cells are plastic, it is dependent on the establishment of certain cell markers, the roles of the transdifferentiated cells were not convincingly shown in other situations. Furthermore, some researchers have questioned the characterization of transdifferentiation of infused cells into neural cells, claiming that the observed transdifferentiation was caused by the confluence of infused cells of bone marrow and cells of the recipient (Figure 2).
BMSCs
It was discovered that BMSCs may be used to selectively generate dopamine neurons. This method initially produces postmitotic functioning neuronal cells with great efficiency even without glial cell interference. The resultant neural cells are subsequently stimulated to become DA neurons. Lipofection of a plasmid encoding a Notch1 Intracellular Domain (NICD) and G418 screening, throughout by feeding of a particular mix of trophic substances and cytokines, results in induction. As a result, in a rodent model of PD, immature adult BMSCs embedded in the striatum promote substantial but not dramatic restoration of the dopamine channel. A clinical trial of correspondent immature transplanting BMSC into PD disease sufferers monitored for approximately 36 months revealed a proportion of symptom relief with no tumor development. While these cells offer benefits over all other stem cells in terms of safety, ease of access and trophic effects but these effects do not persist for a long time and ultimately decrease by the time because of limited survival of cell in-vivo. The adaptation of BMSCs to the in vitro environment, as well as their multiplication activity, varies between species. Generally, rat and human BMSCs can grow persistently in vitro, but primate and mouse BMSCs are prone to manipulation, frequently resulting in the failed introduction of NICD gene via lipofection due to lipofection cytotoxicity. Mostly during stimulation, cells die and this method is a challenge in implementing cell based treatment, therefore more safe and efficient gene insertion is critical for practical application. Reverse transfection using spermine is an efficient technique for delivering plasmid genes, even inside cells. Furthermore, NICD genes that have been introduced are efficiently transcribed and generated as polypeptides inside the cell of primates, rodents and humans BMSCs, with relatively low levels of toxicity. That method is indeed efficient in establishing DA neurons from primate and rodent BMSCs. In HPLC NICD introduction into BMSCs through spermine mediated reverse transfection accompanied by the intake of cytokines effectively stimulates neuronal cells with DA release. As a result, spermine mediated reverse transfection is an excellent alternative technique for inducing DA neurons from BMSCs of different species.
NSCs
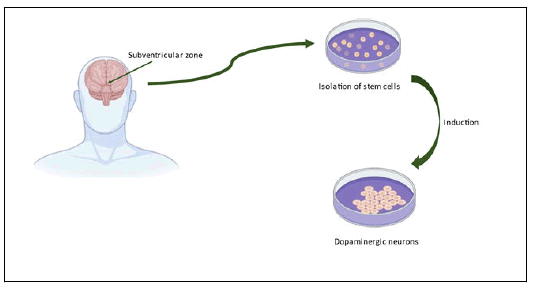
In 1965, Neural Stem Cells (NSCs) were defined as granule cells with such a high percentage of cell proliferation in the brain cortex along with the subventricular zone and hippocampal region and can easily differentiate into astrocytes, oligodendrocytes and neurons too. Before transplantation, NSCs can be stimulated to develop into neural cells in vitro, it was shown that Lmx1a and Msx1 act as progenitor factors, initiating the formation of DA neurons with midbrain identity. Several elements have been demonstrated to induce neural progenitor differentiation into midbrain DA neurons in chick embryos. Such results revealed that Lmx1a and Msx1 play important roles in DA neuron identification and development. NSCs were developed into DA neurons using a five-step technique identical to those used to differentiate ESCs and the morphological features of forebrain DA neurons were established. According to some research overexpressing the transcription factor, ASCL1 was able to recover neurogenesis in human neural progenitor cells, resulting in bigger neurons with more neurites. The discovery of a NURR1 mutation in a PD patient showed that NURR1 performs a regulatory function in the formation of DA neurons. Increased expression of NURR1 was shown to improve the capacity of rodent NSCs to develop DA neurons and sustain in vivo in a rodent model. Experimental research revealed that rodents and humans foetal brain DA neurons grafted to the midbrains of 6- OHDA-lesioned rats thrived in the recipient's brain and alleviated the motor deficits of rats suffering from PD (Figure 3 and Table 1).
| S.no | Study title | Status | Mediation |
|---|---|---|---|
| 1 | MSCs divided into NSCs Used in PD patient. | Recruiting | Umbilical cord derived injection |
| 2 | Phase 2 clinical trial of MSCs and placebo | Recruiting | Placebo and MSCs injection |
| 3 | Using Cell technology for PD therapy | Recruiting | Autologous MSCs injection and placebo |
| 4 | Double blind clinical trial | Recruiting | HB-adMSCs and placebo |
| 5 | MSK-DA01 cell therapy for PD | Recruiting | MSK-DAO1 cell delivery device |
| 6 | Neurologic stem cell therapy | Recruiting | Intravenous-BMSC Intranasal-BMSC |
| 7 | Biological sample collection | Recruiting | Sample collection |
Table 1: Currently recruiting clinical trials for stem cells therapy for parkinson’s disease.
Conclusion
iPSC-derived neurons offer an interesting approach for PD modeling, with both the potential for growth as a clinical treatment option and the development of novel treatments for patients. While iPSC-derived neurons offer great promise in PD, any ongoing clinical techniques should indeed be strongly supported by validated, strong scientific data. Even the loss of a tiny cluster of 7800 DAns in the SNpc can cause significant infirmity in PD sufferers. Although apoptosis can occur due to a variety of hypothesized reasons, neuronal longevity is ultimately required for appropriate neurocognitive function. With no existing treatments for recovering from crucial cell death, iPSCs provide an alternative path to potentially restoring a disease free condition. Because of its capacity to control DAn survival, MSC-secretome has been considered as a possible treatment approach for various neurological conditions, including PD. MSCderived exosomes, together with the protein fraction, are a significant tool and therapy alternative within it. Furthermore, in animal studies, the interchange of genetic material, such as miRNA, via exosomes can stimulate neurogenesis, decrease neurotoxicity and increase healing. Determining the intricacy of MSC-derived exosomes and how their miRNA content connects with cellular and molecular PD pathways is thus critical. Quite a strategy would not only enable the exploration of possible pathways implicated in the illness's recovery processes, but also for the creation of multi targeted methods that might produce potential therapeutic improvements for PD patients.
Acknowledgment
This work has been supported and authored according to the work as research initiatives by Jozbiz technologies.
References
- Braak H, Del Tredici K, Rub U, de Vos RA, Steur ENJ, et al. (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24: 197-211.
[Crossref] [Google Scholar] [PubMed]
- Garcia-Ruiz PJ, Chaudhuri KR, Martinez-Martin P (2014) Non-motor symptoms of Parkinson's disease. A review from the past. J Neurol Sci 338: 30-33.
[Crossref] [Google Scholar] [PubMed]
- Ball N, Teo WP, Chandra S, Chapman J (2019) Parkinson's disease and the environment. Front Neurol 10: 217-218.
[Crossref] [Google Scholar] [PubMed]
- Jakubowski JL, Labrie V (2017) Epigenetic biomarkers for Parkinson’s disease: From diagnostics to therapeutics. J Parkinsons Dis 7: 1-12.
[Crossref] [Google Scholar] [PubMed]
- Arenas E (2002) Stem cells in the treatment of Parkinson’s disease. Brain Res Bull 57: 795-808.
[Crossref] [Google Scholar] [PubMed]
- Klein C, Westenberger A (2012) Genetics of Parkinson’s disease. Cold Spring Harb Perspect Med 2: 008888.
[Crossref] [Google Scholar] [PubMed]
- Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, et al. (2007) How common are the common neurologic disorders? Neurol 68: 326-337.
[Crossref] [Google Scholar] [PubMed]
- Elbaz A, Bower JH, Maraganore DM, McDonnell SK, Peterson BJ, et al. (2002) Risk tables for parkinsonism and Parkinson's disease. J Clin Epidemiol 55: 25-31.
[Crossref] [Google Scholar] [PubMed]
- Winkler AS, Tutuncu E, Trendafilova A, Meindl M, Kaaya J, et al. (2010) Parkinsonism in a population of northern Tanzania: A community-based door-to-door study in combination with a prospective hospital-based evaluation. J Neurol 257: 799-805.
[Crossref] [Google Scholar] [PubMed]
- Okubadejo NU, Bower JH, Rocca WA, Maraganore DM (2006) Parkinson's disease in Africa: A systematic review of epidemiologic and genetic studies. Mov Disord 21: 2150-2156.
[Crossref] [Google Scholar] [PubMed]
- Zhang ZX, Roman GC, Hong Z, Wu CB, Qu QM, et al. (2005) Parkinson's disease in China: Prevalence in Beijing, Xian and Shanghai. Lancet 365: 595-597.
[Crossref] [Google Scholar] [PubMed]
- Tan LCS, Venketasubramanian N, Jamora RDG, Heng D (2007) Incidence of Parkinson's disease in Singapore. Parkinsonism Relat Disord 13: 40-43.
[Crossref] [Google Scholar] [PubMed]
- Willis AW, Evanoff BA, Lian M, Criswell SR, Racette BA (2010) Geographic and ethnic variation in Parkinson disease: A population-based study of US Medicare beneficiaries. Neuroepidemiol 34: 143-151.
[Crossref] [Google Scholar] [PubMed]
- Horsfall L, Petersen I, Walters K, Schrag A (2013) Time trends in incidence of Parkinson’s disease diagnosis in UK primary care. J Neurol 260: 1351-1357.
[Crossref] [Google Scholar] [PubMed]
- Bower JH, Maraganore DM, McDonnell SK, Rocca WA (2000) Influence of strict, intermediate and broad diagnostic criteria on the ageâ?Âand sexâ?Âspecific incidence of Parkinson's disease. Mov Disord 15: 819-825.
[Crossref] [Google Scholar] [PubMed]
- McIntyre CC, Anderson RW (2016) Deep brain stimulation mechanisms: The control of network activity via neurochemistry modulation. J Neurochem 139: 338-345.
[Crossref] [Google Scholar] [PubMed]
- Bloem BR, de Vries NM, Ebersbach G (2015) Nonpharmacological treatments for patients with Parkinson's disease. Mov Disord 30: 1504-1520.
[Crossref] [Google Scholar] [PubMed]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, et al. (1998) Embryonic stem cell lines derived from human blastocysts. Science 282: 1145-1147.
[Crossref][Google Scholar] [PubMed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences